Rep:Mod:mjs281287
Module One: Structure and Spectroscopy
The Hydrogenation of Cyclopentadiene Dimer
| Endo Dimer | Exo Dimer | |
|---|---|---|
| Stretch | 1.2541 | 1.2831 |
| Bend | 20.8279 | 20.5945 |
| Stretch - Bend | -0.8370 | -0.8388 |
| Torsion | 9.5168 | 7.6562 |
| Non-1,4 VDW | -1.5427 | -1.4303 |
| 1,4 VDW | 4.332 | 4.2388 |
| Dipole/Dipole | 0.4486 | 0.3774 |
| Total Energy | 33.9998 kcal/mol | 31.8809 kcal/mol |
From the data in the table the endo form, that is prefered form of the dimer, is thermally less stable (33.9998 kcal/mol) than the exo conformer (31.8809 kcal/mol). The exo form appears to adopt a chair like formation and the endo appears to resemble a sort of boat shape. The torsion value between the two conformers is the biggest factor in why one is more stable than the other. The endo form torsion number is higher than that of the exo. This is due to the boat type shape the endo has formed meaning the strain in the bonds is higher than that of the other.
Another reason why the endo is prefered is due to orbitals. The frontier orbitals on the two cyclopentadienes, before dimerising, have the correct symmetry at the front and back of the molecule so that two are able to form two new sigma bonds and the other three orbitals are there creating intereactions between the two cyclopentadiene rings, not to form bonds, but to make sure the endo product is formed.
Since the endo arrangement is more thermally unstable but is the prefer conformer for the dimer this therefore means the actual process of dimerisation is kinetically controlled. This is proven by the fact that if cyclopentadine, the monomer, dimerizes at low temperatures ie if left over night it would form the dimer.

When hydrogenating the preferred endo dimer, structure four is formed first due to it being more thermally favoured to hydrogenate that double bond instead of the other shown in struture three. Examing the whole table the values are pretty similar except for the bend in the overall molecule. The bend in four is lower than that of in three. A possible reason behind this is that the favoured bond to hyrdrogenate would become longer in length and so reliving the bit of added strain felt by the bridging carbon when a double bond is in place.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Derivative of prolinol |
For the derivative of prolinol the carbonyl with respect to the aromatic ring is fairly planar. The dihedral angle between the oxygen and the carbon at the top of the aromatic ring is 25.2o therefore it is nearly within the plane of the ring and only sligtly out. This means there can be hydrogen bonding stabilisation between the oxygen in the carbonyl and with the hydrogen pointing in the same direction on the aromatic ring.
Pyridinium ring |
The pyridium ring carbonyl is slightly different in that is no longer within the plane of the aromatic rings. The dihedral angle is now 42.8o meaning it is pointing into the plane. This might be due to the fact of the extra two methyl groups attached to the nitrogen (amide group) within the cycloheptane ring (in structure 7) instead of just the pyrrole type ring. The two methyl groups have their hydrogens readily available to interact with the carbonyl oxygen making the overall molecule more stable. This extra hydrogen interaction forces the oxygen to move out of the plane with the aromatic rings to adopt a more stable arrangement.

The data above renforces the fact about the stability of the molecules. Struture seven is more stable than structure five this is becuase of the extra hydrogen bonding seen in 7. However, the extra aromatic rings help to stabilise the whole strucutre as well.
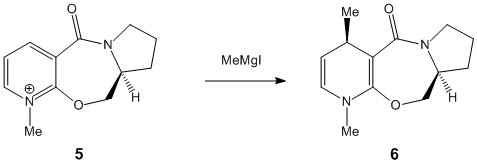
When the derivative of prolinol is reacted with the Grignard reagent the methyl is added so that it comes out of the plane as shown in struture six, to the right. This is probably due to the magnesium coordinating with the carbonyl oxygen which inturn allows the methyl group to add in the very stereospecfic nature shown in the reaction scheme to the right (structure five to six). When the methyl group is added to the pyridine ring the magensium forms an enolate with the oxygen. Since the seven membered ring is so rigid there is no other way the carbonyl can align itself and so the methyl has to add the way shown.
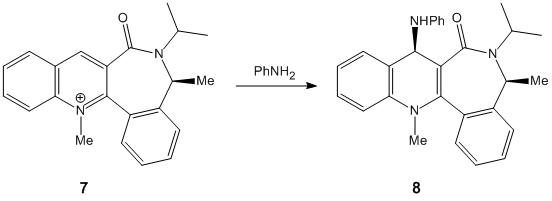
The NHPh adds with the same geometry as the methyl group in the previous example but this time it is for a different reason. The reason for the stereochemistry is the loan pairs on the nitrogen, just the one, and on the oxygen, two. As the nitrogen comes into the attack the aromatic ring there is only one line of attack it can follow. With the oxygen out of the plane of the overall molecule the nitrogen must come from the opposite side as to reduce repulsion between the two sets of loan pairs. This consequently minimises the strain felt on the molecule by adopting the specific geomerty shown in structure eight.
Taxol
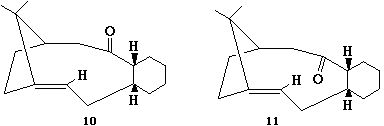
From looking at the table it is obvious to see that structure ten, when the carbonyl group is pointing up is far more stable than when it is pointing down as in structure eleven. The energy of eleven is more than double than that of ten. The biggest contribution to the unstability is the bend within the molecule. The bend within structure eleven is five times than that of in ten. On rotating the Jmol pictures of the molecules, the two carbons to which the carbonyl group is attached to each is more a less straight line therefore it is not at its normal bond angle of 120 degrees and trigonal planar geometry. This makes strain within the molecule making it more unsatble in terms of bend.

Taxol intermediate - carbonyl pointing up
Derivative of prolinol |
Derivative of prolinol |
Taxol intermediate - carbonyl pointing down
The hydration of this intermediate (when the carbonyl points up) is a very slow process. One of the reasons is becuase the total energy of the molecule raises when it becomes a single bond therefore the new molecule is thermally unstable with respect to starting intermediate and so it is not thermally favourable to hydrogenate. Plus another reason is that the double bond keeps the structure quite rigid and when the double bond is hydrated the bind length becomes longer and the overall structure becomes "floppy" and the oxygen is not interacting with as many hydrogens as before therefore less hydrogen bonding.
Peptide
| Structure | Jmol | Total Energy (kcal/mol) | |||
|---|---|---|---|---|---|
| Axial Isomer on 13 |
|
16.9394 | |||
| Equatorial Isomer on 13 |
|
13.1254 | |||
| Axial Isomer on 14 |
|
13.0135 | |||
| Equatorial Isomer on 14 |
|
11.7367 |
In both cases, structures thirteen and fourteen, the more favoured form of the respective molecules is when the amide group is equatorial to the ring instead of when its axial. This confirmed from the difference in total energy of the two different isomers of each structure in the table to the right. In structure 13 the energy difference is about 1.4 kcal/mol and for structure 14 it is 3.8 kcal/mol. This difference between the two structures energy difference is vital later when their relative rates of reaction are compared to each other. The equatorial in both cases is more stable becuase of the interactions, hydrogen bonding, felt between the nitrogen (amine group) and the oxygen (in the amide and the hydroxy group) and the surrounding hyrdogens on the extra groups and with the ring ones as well. As shown in the Jmol for the equatorial amide in 13 the the amine group (NH2) has its hydrogens interacting with the hydroxy oxygen and the methylene in the amide is interacting with the carboxyl oxygen so is the hydrogen equatorial to the hydroxy group.
When the amide group is equatorial the reaction shown in reaction scheme can occur. However, this occurs in thirteen far quicker than in fourteen. This is becuase the nucleophilic attack between the amine and the hydroxl group can occur in thirtenn whereas in fourteen the amide group must first become axial by ring flipping. This process requires energy input first and so the rate is slower than the one in the other example. The amide groups must become axial first so that it can have the appropriate angle of attack at the hyrdroxyl group for the intramolecular nucleophilic substitution.
Dichlorocarbene
| Orbital | Picture | Energy(J) |
|---|---|---|
| Homo | 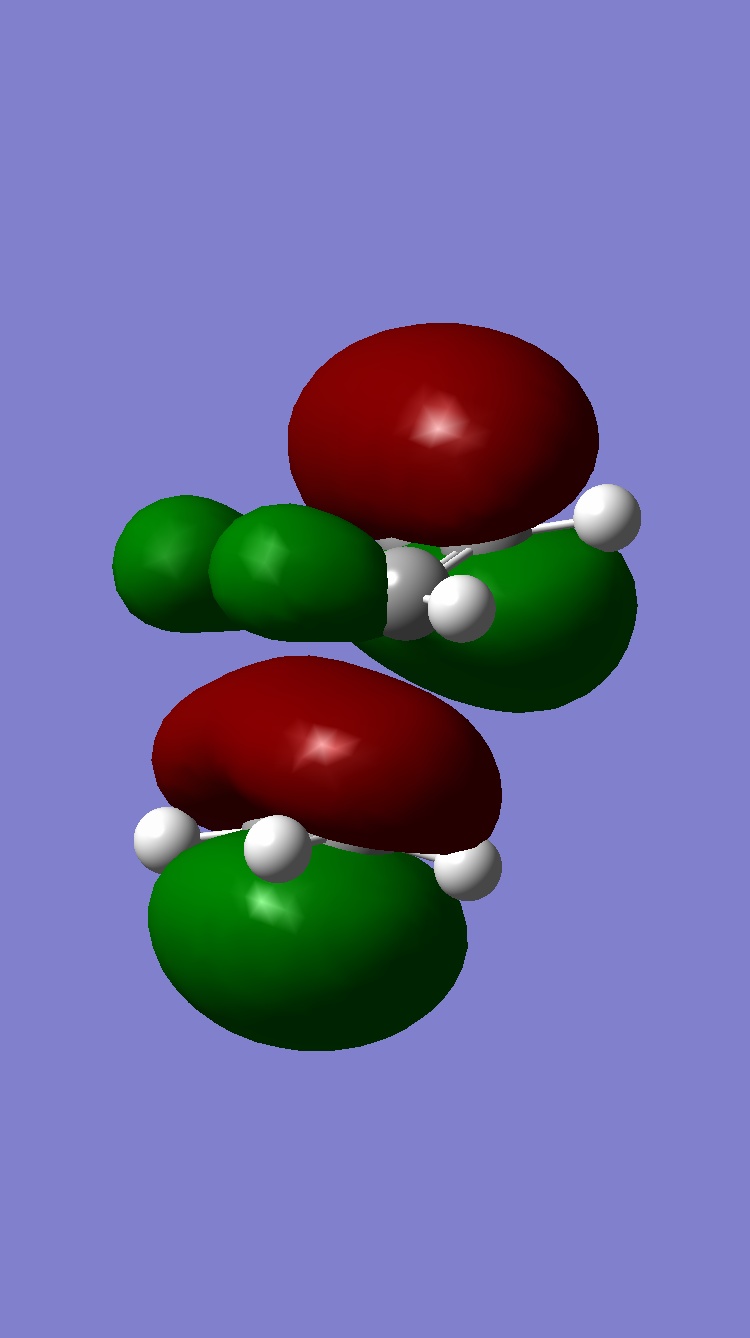 |
-0.337 |
| Homo-1 | 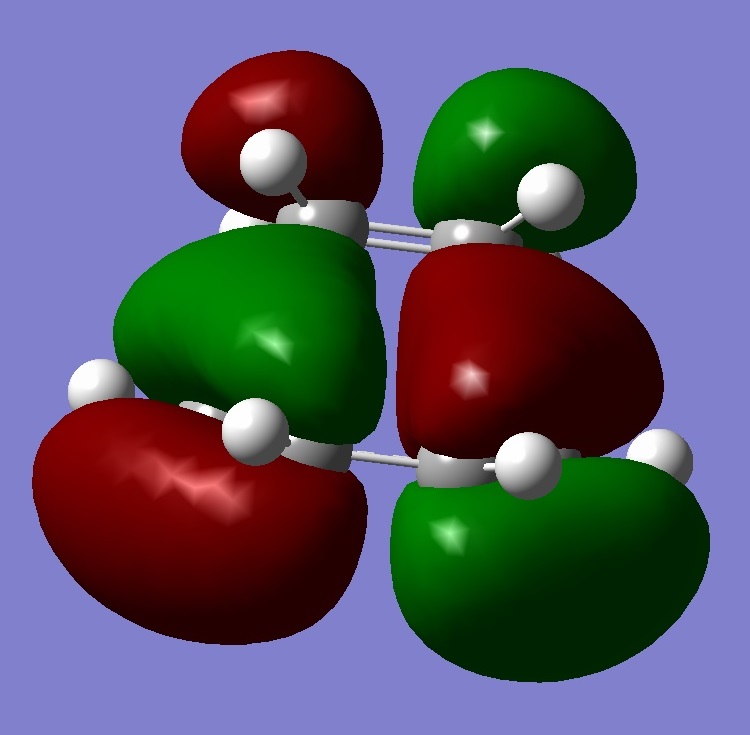 |
-0.335 |
| Lumo |  |
-0.156 |
| Lumo+1 | 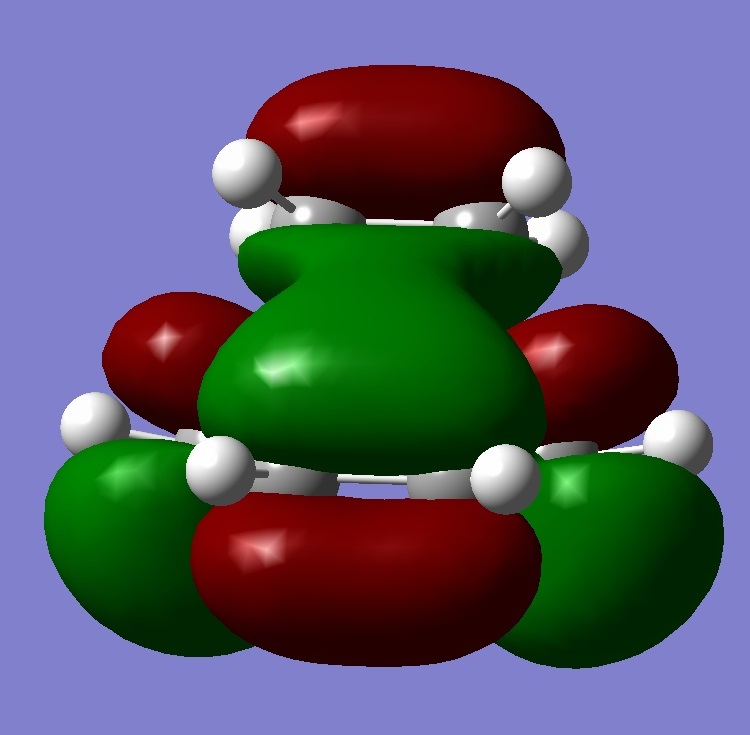 |
-0.153 |
| Lumo+2 | 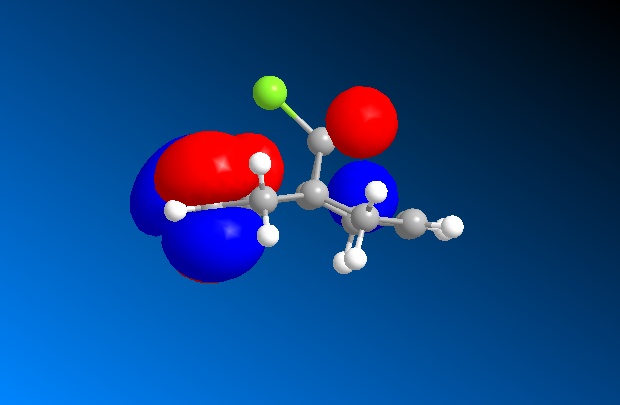 |
-0.078 |
Looking at all the orbitals above, the energies are all negative meaning they are all bonding orbitals with or without being filled up. The HOMO has a large cluster of electron density around the chlorine atom on the bridging carbon. It appears the electron density is coming from an interaction from the chlorine and the hyrdogens attached to one of the six memembered rings. This therefore means, with this high electron density around the chlorine, the whole molecule is susceptible to electrophilic attack with the chlorine acting as a leaving group.
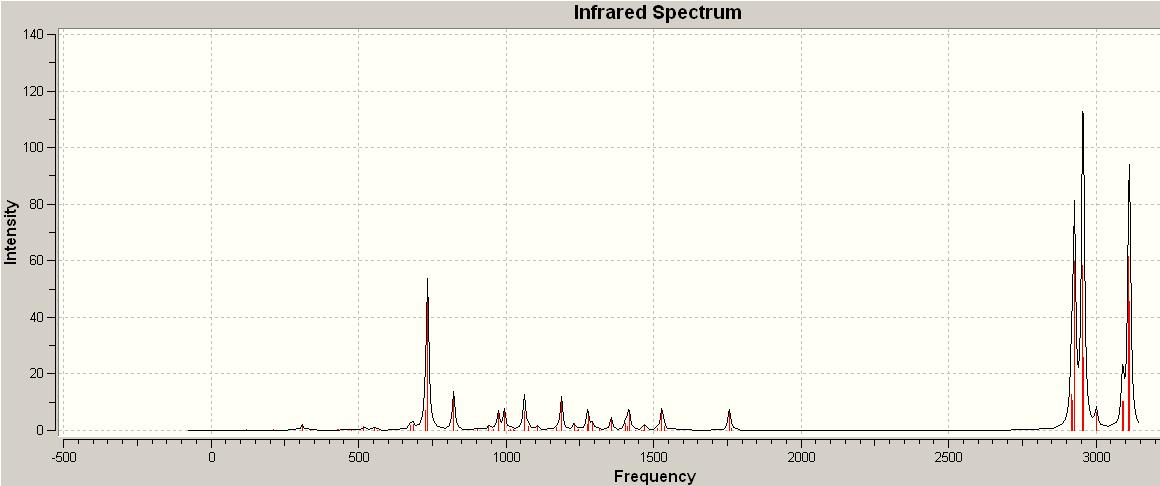
With the normal strecthin frequency for a carbon carbon double bond being between 1620-1680Hz, the stretching frequency in both the non hyrdrogenated molecule tweleve and the hydrdrogenated one the frequnecy is slighly higher at 1756.62Hz and 1762.99Hz for the non hydrogenated one and at 1760.17Hz for the other. This is about 100Hz higher than normal. Also there is only one stretching frequency for the hydrogenated version and two for the other one. The spectrum above is the one for the non-hydrogenated molecule. Looking at the relative intenisties the carbon carbon double bond stretch is very low and the higher intensities are at the 3000Hz and 600Hz, where the carbon hydrogen bond stretching/vibrating occurs. The carbon chlorine bond stretch occurs at 974.289HZ for the non-hydrogenated molecule.
The image below is the spectrum for the hydrogenated version of molecule 12. The stretching frequency as stated above for the carbon carbon double bond is pretty similar at 1760.17Hz however there is only one. the carbon chlorine stretch though is over 100Hz smaller than that in the one above, at 833.608Hz. The hydration of the molecule must affect the vibrations of the molecule by changing the overall geomoetry and shape of the entire molecule.
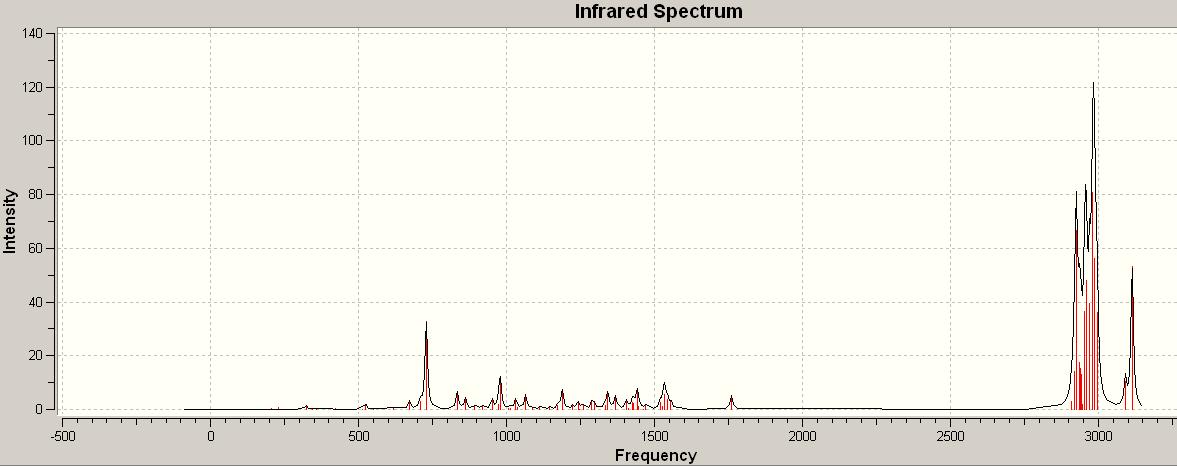 The overall intensity, of the carbon carbon double bond, in this spectrum is lower than in the other. This could e due to the fact that there is only one frequency vibration contributing to the intensities. The high intensity are still around the same points due to the fact of the carbon hydrogen bonds. If anything the intensity is larger decause of the extra carbon hyrdrogen bonds formed from hydration.
The overall intensity, of the carbon carbon double bond, in this spectrum is lower than in the other. This could e due to the fact that there is only one frequency vibration contributing to the intensities. The high intensity are still around the same points due to the fact of the carbon hydrogen bonds. If anything the intensity is larger decause of the extra carbon hyrdrogen bonds formed from hydration.
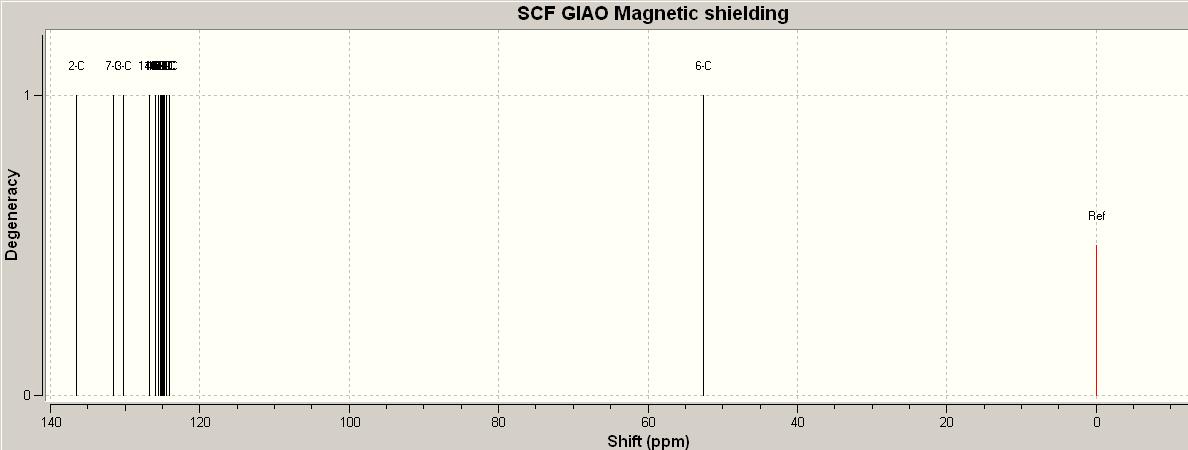
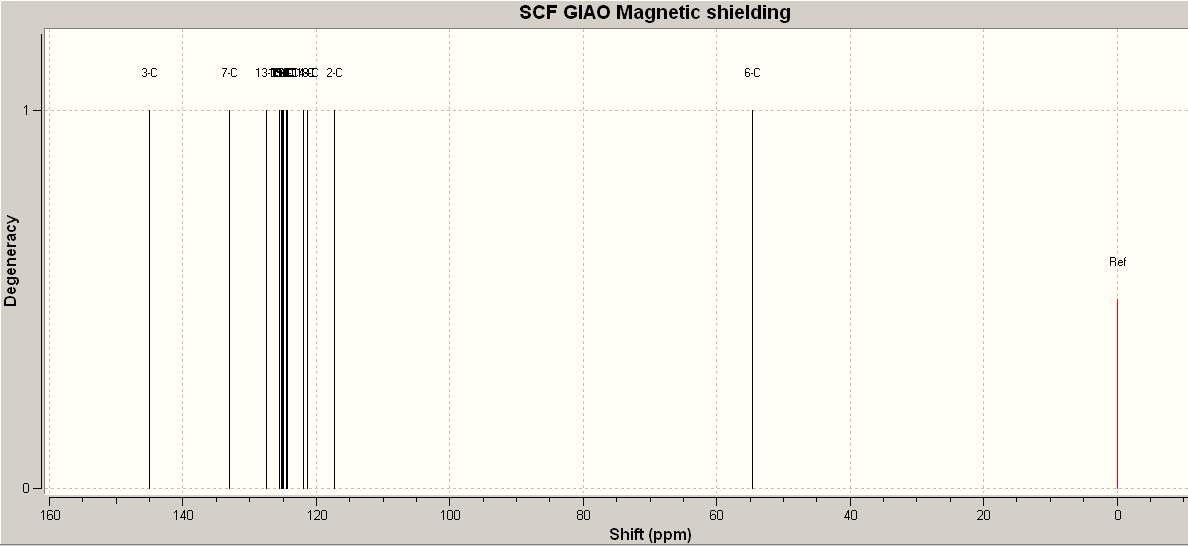
Click Chemistry

Looking at the two prdicted NMR's from Gaussview through chemistry scan, they are pretty similar their chemical shifts. However the second one has the peaks all shifted higher in ppm ie there are peaks above 140ppm. Whereas the top one has the peaks below 140ppm. Comparing these spectrum to the actual experimental data it is clear that the top one matches the data the closest by having no peaks over 140ppm and lie within in the range of the experimental chemical shifts. Also they chemical shifts are more bunched together which coincides with the data in the table becuase the chemical shifts are all close together. Therefore the isomer that is closest is the one where there is carbon between the two neighbouring aromatic rings and they are joined adjacent to each other.
Link for the published NMR data with the corresponding carbons that create the shifts.
http://hdl.handle.net/10042/to-1018
References
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 ) and 95, 3051 for another nice example of atropisomerism.
- M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
- B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s