Rep:Mod:mjs1987i
Inorganic Module 2: Bonding
Information about BCl3
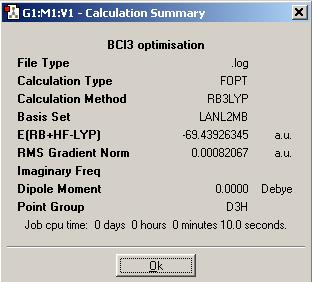
On the right are all the values from the gaussview calculation carried out on BCl3.
The bond angle between Cl-B-Cl is 120ο, therefore the molecule is trigonal planar.
The bond distance between Cl-B is 1.870 Angstroms.
Vibration Analysis
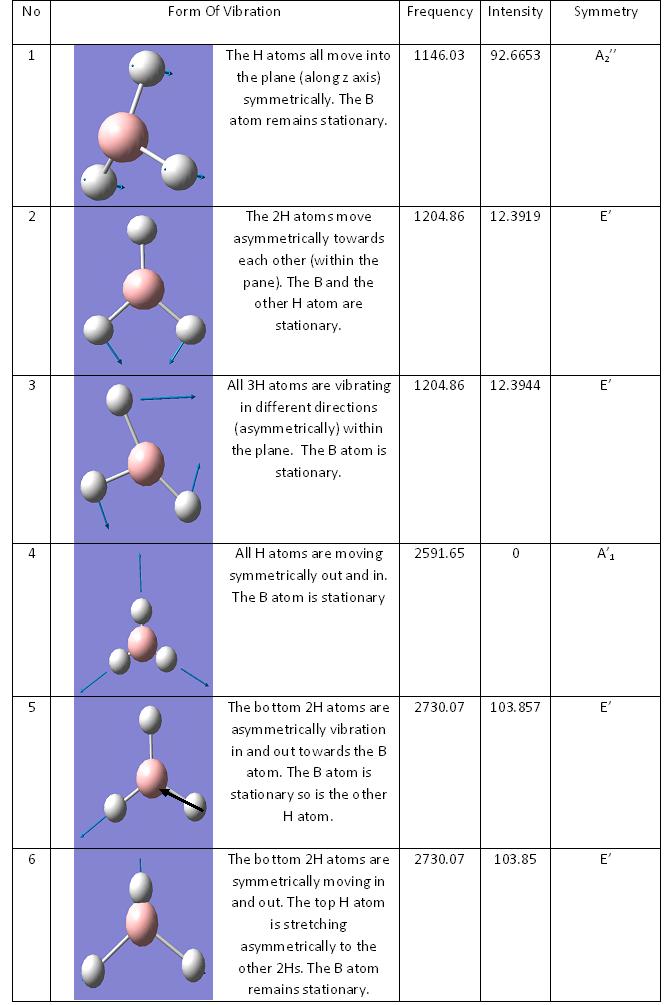

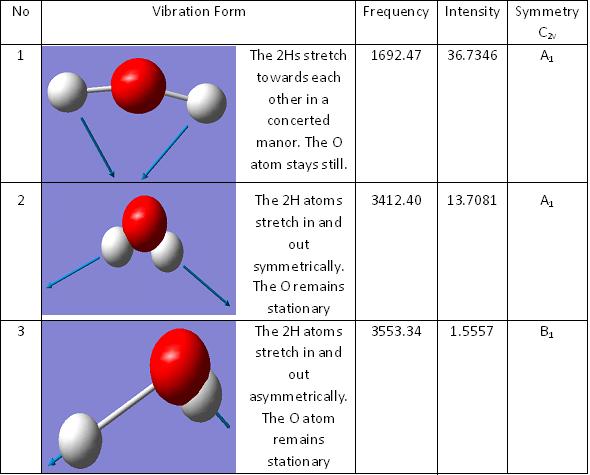
Looking at the spectrum there is only three peaks on the IR whereas in the table above there are 6 frequencies recorded. The cause for this is that there are two sets of two vibrations that have the same frequency and therefore shown as two peaks instead of four. There is another stretch frequency but there is no intensity for this. This is becuase there is no change in dipole and therefore it does not appear on the spectrum.
In the spectrum there is two peaks that have two separate vibrations contributing to it and there is one that creates its own peak.
Molecular Orbitals
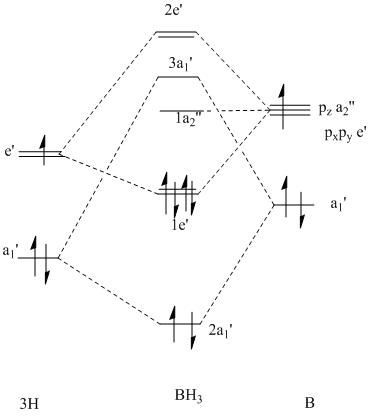
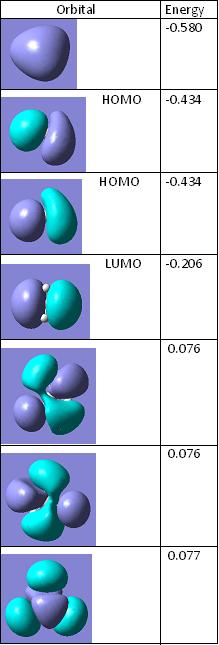
To start with the 1s orbital on both MO diagrams is being ignored becuase it is too low in energy to be an affect to anything.
The picture on the left is the drawn MO on the diagram and the table on the right is the lifted images from Gaussview. They both match very well, there are two sets of dengenerate orbitals in both. One set is bonding and is also the HOMO and the other is the anti-bonding orbitals of the bonding ones. The non bonding orbital in the drawn version contains no electrons making this the LUMO as well. This correlates to the Gaussview version by being higher in energy than the HOMOs but still negative in energy meaning it is still in the bonding section of the diagram.
The difference between the two is that the top two antibonding orbitals, the degenerate one and the 2s are switched around in the drawn version to that in the gaussview. This therefore gives rise to the fact that qualitative MO diagrams are very useful and accurate and the only flaw is that of the postioning of the anti-bonding orbitals to each other. It is hard to judge which one is destabilised more with respect to the other.
Small Molecule
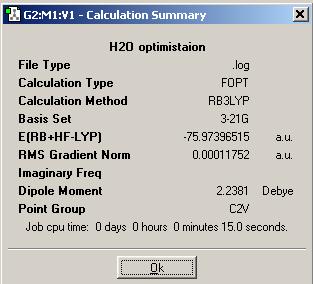
The small molecule that was picked was H2O.
On the right are all the values from the gaussview calculations carried out on H2O.
The bond angle between H-O-H is 103.5ο
The bond distance between H-O is 0.996 Angstroms.
Ammonia
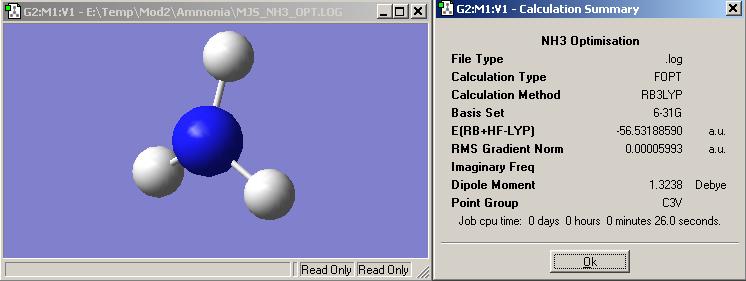
Using the basis set B3LYP with 6-31G the optimised structure achieved is shown to the left.
The symmetry of ammonia when it is in its tetrahedral based structure is C3v.
The job time for this operation was 26s.
The energy of this molecule was -148427 KJ/mol
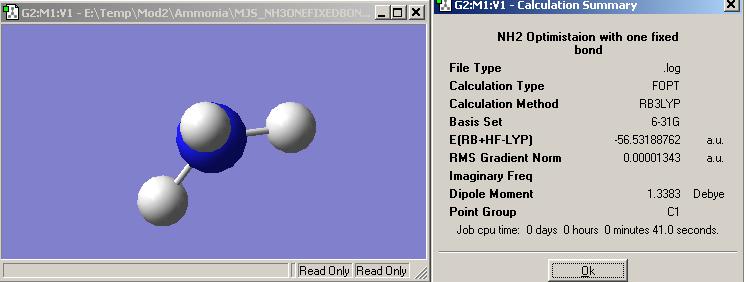
Fixing a N-H bond length to 1.01 Angstroms and using the same basis set and pseudo-potentials as before the optimised structure that is created is shown on the left plus all its relevant data.
The job time for the optimisation was 41s.
The point group for the ammonia is now C1.
The energy for this molecule was -148427 KJ/mol
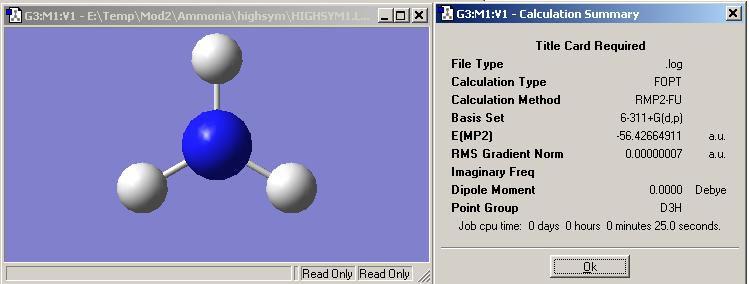
Once again using the same basis set and pseudo-potentials optimisation was calculated and the information shown on the left.
The job time for the optimisation was 16s
The point group for the ammonia is now D3h.
The energy for this molecule was -148148 KJ/mol
For C1 and C3v the symmetry has had no effect on the final structure becuase both have the tetrahedral based structure. However when the point group is D3h the final structure is now trigonal planar and therefore planar.
The change in symmetry has more effect on the time is takes for the optimisations to run. The slowest is C1, where one of the bonds is fixed, then C3v is the second slowest and the fastest is the D3h. The reason behind this is the order of symmetry it has. The higher the symmetry the molecule has been classed with the quicker the calculation will be. This is due to the fact that the computer relises that there are three N-H identical bonds (in the D3h) and therefore only needs to carry out one calculation compared to the three in the lower symmetry cases.
A molecule when being optimised can break symmetry. This is because when the geometry is being optimised the structure is moving to its most stable form and if that happens to be a different point group with different symmetry then it will break it. However if the symmetry is locked in, the higher the better, then it will not break symmetry and so optimise within its constraints.
The higher the symmetry you set the quicker the caluclations take due to the fact it does not have to do every single calculation because for example it can do one for four bonds. However a drawback to having high symmtery will lock in the molecule and not necassery optimise it to its lowest energy.
The lowest energy out of all the different symmetries is the ammonia with D3h point group. The energy difference between the two is important becuase its the energy gap that must be overcome for the molecule to invert and the hyrdrogens to flip. The energy gap must therefore be not too large otherwise the flipping will not occur.
The energy difference between the C3v and D3h is 20 KJ/mol. Comparing this to the experimental value of 24.3 KJ/mol, the calculated value is only 4KJ out. This is probably due to the fact that the basis set used is not high enough to give an accurate eneough answer. Therefore a higher calculation must be used.
Method

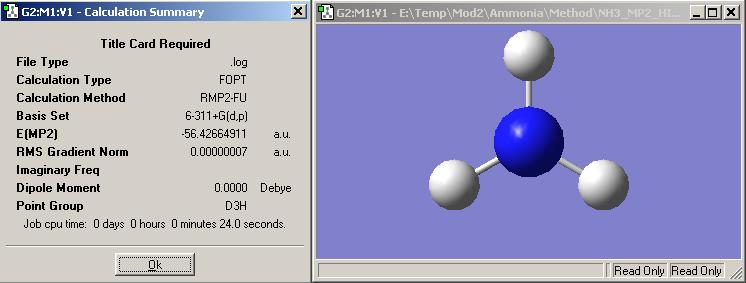
Comparing these calculations on the left to the lower levelled ones run these one take a longer time. This is becuase we are using a higher basis and pseudo-structure for the optimisation. However, using a higher basis set means the calculation is more accurate and this is shown in the energy outputs. With the higher basis set the energy is higher than that of with the lower basis set. The energy gap is smaller with the more accurate basis set because of the higher accuracy obtained.
The energy difference between the D3h (-148148KJ/mol) and the C3v (-148169KJ/mol) is 20.5KJ/mol. This is only a difference of 4KJ/mol between the caculated results and the experimental result of 24.3KJ/mol. This is ok but a way of improving this would be to carry out a longer calculation with a high basis set to get a closer energy to actual experimental results.
Vibrations


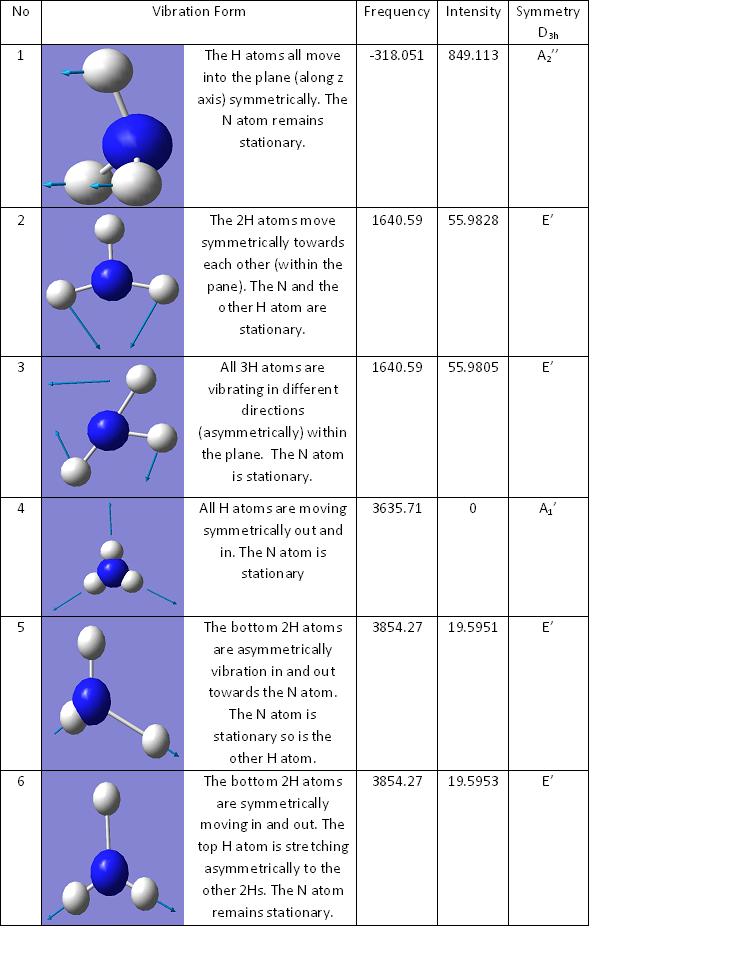

For the D3h ammonia there is only one vibrational frequency thats is negative. For the C3v ammonia there are no negative frequencies in the vibrational analysis. The first four vibrations of both C3v and D3h match up more or less. The only discrepancies to note is number four stretch (where all the hydrogens symmetrically vibrate causing no overall dipole change) where for C3v the intensity for the vibration is not zero which it should be. This indicates that there must be a very slight change in net dipole causing an IR stretch to be recorded but since it is so small it is negliable. Another difference is with number two stretch where the actual vibration is slightly different between the two. In C3v the top H is ever slightly vibrating into the plane whereas on D3h it is completely stationary.
Comparing the computed frequency bands with the major absorption bands located at http://en.wikipedia.org/wiki/Ammonia_(data_page)#cite_ref-0 they are within the same area as expected. The two smaller peaks on the computed spectrum (at roughly 1650Hz and 3800Hz) are in the correct region for the major absorption. However, the largets intensity (at about 450Hz) is a bit lower than expected. The experimental one is at 950Hz. All the frequencies though positive and so this confirms that the C3v ammonia structure is the ground stat structure.
In the D3h transition state the negative frequency is at -318.051Hz. Frequency one, when the H atoms are stretchin behind the N, which is remaining stationary, is the frequency that follows the reaction pathway. This is becuase in going form one C3v to the other, where the hydrogens have inverted themselves, the ammonia goes through a D3h symmetry. The closest vibration to this is number one, all the others are with either only two hydrogens vibrating or all of them within the plane.
Trans and Cis Isomer
Trans
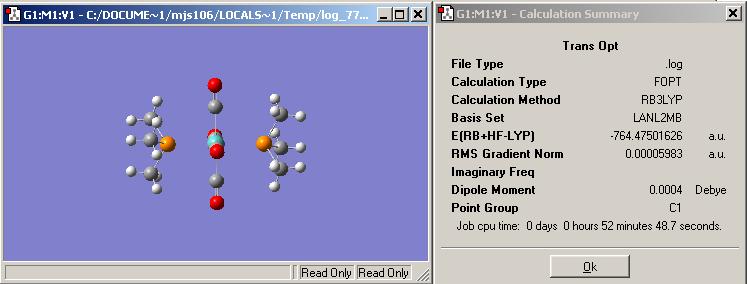
First optimisation of the trans complex using the simple basis set and pseudo-potential
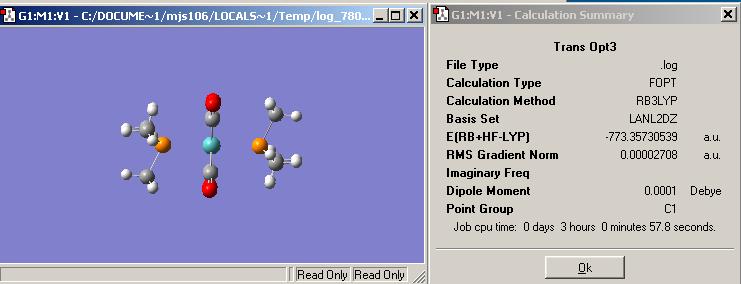

Second optimisation of the trans complex using a more complicated basis set and pseudo-potential. I was unable to post my output file to D-space.
Comparing the two orientations of the phosphoruses to each other, they are in a staggered arrangement to one antoher at either end of the molibdenium centre. With respect to the molibdenium centre the methyl groups attached to each phosphorus are pointing towards the corners of a tetrahedral. Comparing the phosphorus, and its substituents, alignment with the rest of the molecule they are both in a eclipsed style geometry with the CO ligands. It is possible as well that the hydrogens are interacting with the oxygens therefore stabilising the whole transition complex.
URL for the frequency calculation on "D-Space" for the Trans isomer: http://hdl.handle.net/10042/to-1017
Cis
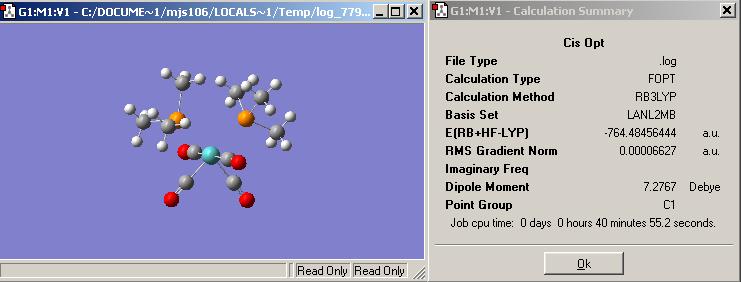

First Optimisation of the cis isomer using a simple basis set and pesudo-potentials
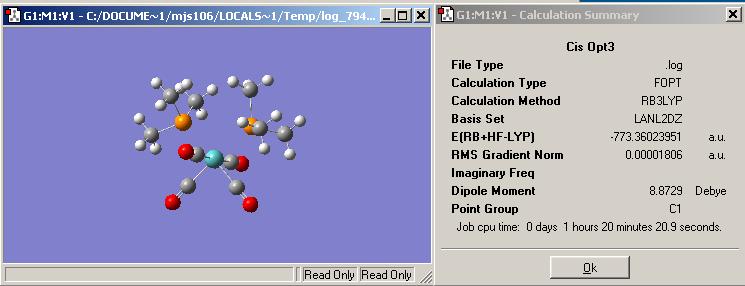
Final Optimisation of the Cis isomer using a more complex basis set and pseudo-potentials. http://hdl.handle.net/10042/to-1055 is the link to "D-Space" for the finalised geometry.
When the molecule is rotated so that one of the phosphorus atoms is behind the other the methyl groups bonded to them are in a staggered formation to reduce steric hinderence becuase they are cis to one another. The phosphoruses in relation to the molibdenium central atom are in a staggered like geometry and also an elcipsed type. There is also possiblilty of the final geometry being stabilised by hydrogen bonding between the methyl groups on the phosphorus and the oxygen on the CO ligands.
URL for the frequency calculation on "D-Space" for the Cis isomer: http://hdl.handle.net/10042/to-1016
The two geometries are different due to the fact that they one is cis and the other is trans. However they are similar due to the fact that the phosphoruses are both staggered with respect to the each other in both cases. And eclipsed with respect to the the molibdenium centre and the CO ligands. The trans isomer probably undergoes more hydrogen bonding than the cis becuase of the position that they are in and have chance to stabilise more oxgygens that the cis isomer.
The two IR spectrums are mostly similar as in they both have all the large intensity peaks in roughly the same place as each other, especially the largest one at about 1600Hz. However the cis spectrum has a lot more smaller peaks on the spectrum compared to the trans. This probably due to the fact that with the trans there are not as many vibrations due to the fact that the molecule is highly symmetric.
The distance between the molibdenium and the phosphorus in the trans isomer is 2.57 Angstroms compared to that in the cis which the distance is 2.65 Angstroms. The trans distance is shorter. Comparing these distances with a transtion metal complex containing Mo-P, 2.56 Angstroms, and Mo=P, 2.21 Angstroms, bonds[1] the trans isomer is the closest to having a single bond distnace between the molibdenium and phosphorus and that there is no double bond charcter in either the trans or cis.
The trans isomer is more stable than the cis. This is because the phosphoruses are closer to the molibdenium centre than the cis due to the steric hinderence felt between the two phosphoruses is higher when at cis and so are further away from the molibdenium centre. This therefore reduces the effect of hydrogen bonding which compromises stabilizes the overall compound.
Ammonia Borane

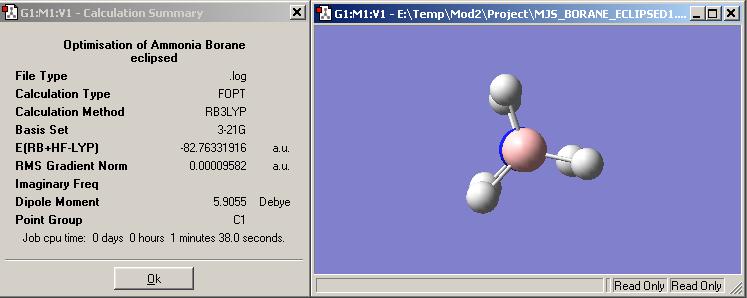

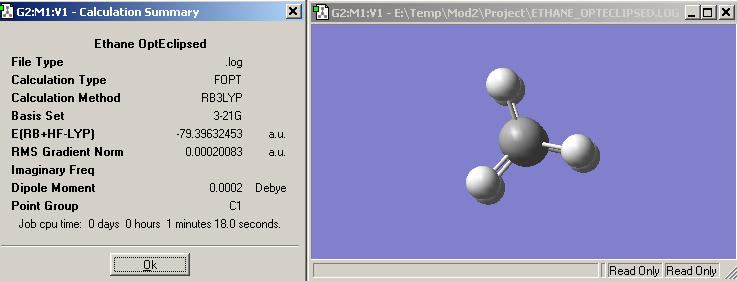


The overall geometry for ammonia borane is when it is staggered and belongs to the point group C3v. This is due to steric hindrence and repulsion being reduced when the hydrogens are staggered instead of eclipsed. In the eclipsed form the hydrogens would repel each other, not my much becuase they are fairly small, but enough for the staggered geometry to be favoured. This is backed up by the difference ine enrgy between the two conformers. The staggered conformer is lower in energy, not by much, than the eclipsed form.
When running the calculation for the eclipsed one the point group became C1 and no C3. When the point group was fixed at C3 and the calculation run the geometry of the shape whould come back as the staggered form. Therefore it can be concluded from this that the staggered arrangement is preferred.
Compairing the energy difference between the staggered and elcipsed form of ammonia borane and the two different conformers of ethane it shows that energy gap for ethane is larger than that of ammonia borane. The energy difference for both is very small however ethane is about 0.01 hartree compared to 0.001 hartree for ammonia borane. This smaller change in energy for the borane compound is probably down to the different bond lengths between the carbon-carbon bond (1.56 Angstroms) to the ammonia-borane bond (1.67 Angstroms).
Opening up the two thumb photos, that contain the energy of the startin materials to make ammonia borane, it is obvious that making ammonia borane is favourbakle becuause it is lower in energy than both reactants. NH4Cl is the closest in energy whereas NaBH4 is a far from close. Also with hydrogen gas being given off as a by product makes the whole reaction energy favourable with the bonus of the reaction going through to completion
References
- Javier Sa´nchez-Nieves, Brian T. Sterenberg, Konstantin A. Udachin, Arthur J. Carty 'The reactivity of terminal chloroaminophosphido ligands towards metal carbonyl complexes. Formation of m2- and m3-phosphinidene clusters' DOI:10.1016/S0020-1693(02)01558-X
2 http://en.wikipedia.org/wiki/Ammonia_(data_page)#cite_ref-0
- ↑ 1
