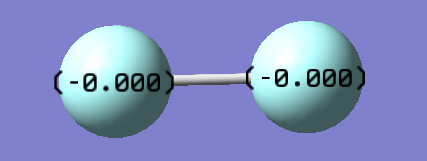Rep:Mod:mjb216
Matt's Wiki page
Ammonia
Ammonia Molecule |
| Bond Length (N-H) | 1.01798 Å |
| Bond Angle (H-N-H) | 105.741 (degrees) |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy - E(RB3LYP) | -56.55776873 au |
| RMS Gradient | 0.00000485 au |
| Point Group | C3v |
The experimental bond length of Ammonia is 1.012 Å[1]. This is very similar to the value generated from the computational calculations.
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy = -5.986271D-10
The optimisation file is liked to here
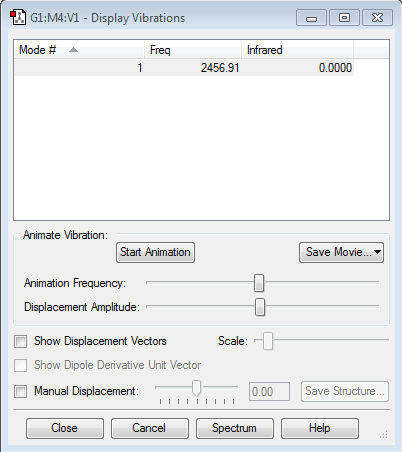
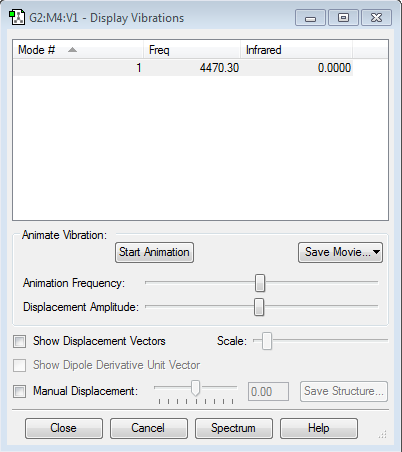
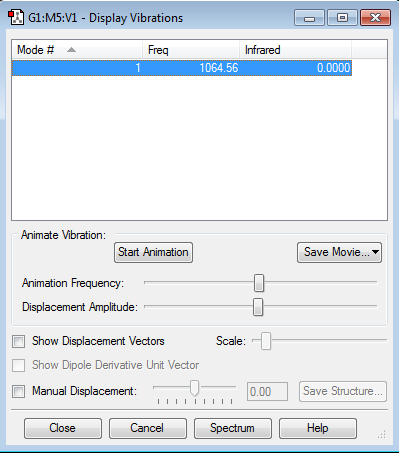
Frequency Analysis
Using the 3N-6 rule, you would expect 6 vibrational modes. Modes 2 & 3 are degenerate and modes 5 & 6 are also degenerate. Modes 1, 2 & 3 are bending. Modes 4, 5 & 6 are stretching. Modes 4 is highly symmetric. Modes 1 is known as the 'umbrella' mode. In an experimental IR spectrum of gaseous ammonia 2 peaks would be present. This can be seen from the intensities of the vibrations, the first mode is very intense, modes 2 & 3 are degenerate, with a reasonable intensity.
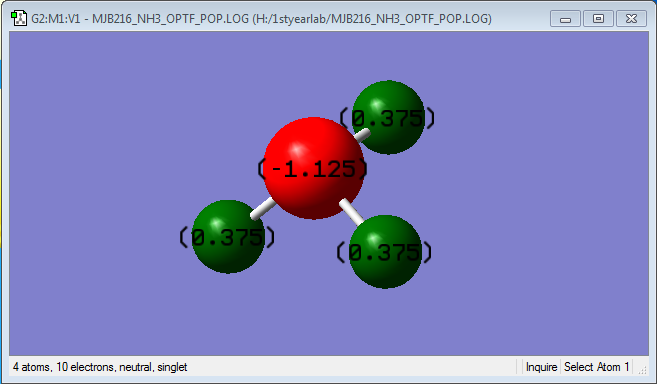
Charge Distribution
As Nitrogen is a more electronegative atom than Hydrogen, it would be expect that the nitrogen atom would withdraw some electron density from the N-H bonds towards it, hence giving it a negative charge.
Nitrogen
Nitrogen Molecule |
| Bond Length | 1.10555 Å |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -109.52412867 au |
| RMS Gradient | 0.00008380 au |
| Point Group | D∞h |
Item Table
Item Value Threshold Converged? Maximum Force 0.000145 0.000450 YES RMS Force 0.000145 0.000300 YES Maximum Displacement 0.000045 0.001800 YES RMS Displacement 0.000064 0.001200 YES Predicted change in Energy = -6.585141D-09
The optimisation file can be found here
Frequency Analysis
The fact that there are no negative energies shows that the molecule has been fully optimised.
Hydrogen
Hydrogen |
| Bond Length | 0.74247 Å |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -1.17853929 au |
| RMS Gradient | 0.00013423 au |
| Point Group | D∞h |
Item Table
Item Value Threshold Converged? Maximum Force 0.000232 0.000450 YES RMS Force 0.000232 0.000300 YES Maximum Displacement 0.000305 0.001800 YES RMS Displacement 0.000431 0.001200 YES Predicted change in Energy = -7.091616D-08
The optimisation file can be found here
Frequency Analysis
The fact that there are no negative frequencies show that the molecule has been fully optimised.
Haber–Bosch process
N2 + 3H2 -> 2NH3
| E(NH3) | -56.55776873 au | ||||
| 2*E(NH3) | -113.11553746 au | ||||
| E(N2) | -109.52412867 au | ||||
| E(H2) | -1.17853929 au | ||||
| 3*E(H2) | -3.53561787 au | ||||
| ΔE = 2*E(NH3) - [E(N2) + 3*E(H2)] | -0.05579092 au | -146.479071618 KJ/mol | -146.48 KJ/mol (2.dp) |
The Ammonia product is more stable as it has a very exothermic formation energy indicating that it is lower in energy than the reactants. The formation enthalpy of Ammonia is -10.93kcal.[2], which equates to 45.76 KJ/mol. As we have formed 2 moles of NH3 in this reaction the ΔE = 91.52 KJ/mol. There is quite a large difference between the literature value and the value from the optimisation. This is because the computer calculates the energies at 0K, whereas the formation enthalpy is measured at 298K. This would cause the difference seen.
Fluorine
Fluorine Molecule |
| Bond Length | 1.40298 Å |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -199.49825220 au |
| RMS Gradient | 0.00000046 au |
| Point Group | D∞h |
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000001 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy = -7.876732D-13
The optimisation file can be found here
Frequency Analysis
The fact that there are no negative frequencies shows that the molecule is fully optimised. As Flourine has no dipole moment, it will have no intensity in the IR spectrum.
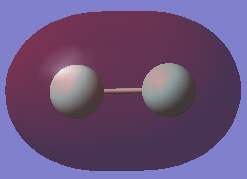
Charge Density
The picture above clearly shows that there is no difference in charge on the two Fluorine atoms. This is because both atoms are the same (Fluorine), and so the electrons will be experiencing the same effective nuclear charge from both, and hence have equal attraction between them. Another way of looking at it is that both atoms have the same electronegativity (as they are the same atom) and so neither will attract electron density more.
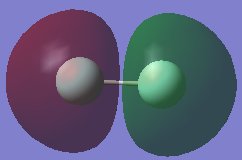
Molecular Orbitals Of Fluorine
Methanal (Formaldehyde)
Formaldehyde |
| Bond Length (C=O) | 1.20661 Å |
| Bond Length (C-H) | 1.11054 Å |
| Bond Angle (H-C-H) | 115.204 (degrees) |
| Bond Angle (O-C-H) | 122.400 (degrees) |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -114.50319935 au |
| RMS Gradient | 0.00004448 au |
| Dipole Moment | 2.1823 (debye) |
| Point Group | C2v |
The experimental bond length of F2 is 1.41 Å.[3]. This is very similar to the computational value calculated.
Item Table
Item Value Threshold Converged? Maximum Force 0.000052 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000142 0.001800 YES RMS Displacement 0.000092 0.001200 YES Predicted change in Energy = -1.371199D-08
The optimisation file can be found here
Charge Distribution
The charge distribution shows that the oxygen has withdraw electron density from the molecule onto itself, giving it a slight negative charge represented by the red colour in the picture. The carbon is slightly positive due to a lack of electron density around it. The blue arrow shows the dipole moment.
Molecular Orbitals
 |
 |
This shows the HOMO & LUMO of the C=O bond. The antibonding region over the Carbon is much larger than that on oxygen. This would explain why during nucleophilic attack of the carbonyl group, the nucleophile always attacks the carbon atom and not the oxygen[4]. The reason that the antibonding orbital is bigger is due to Carbon's atomic orbitals being higher in energy than oxygen's, due to the orbitals being less contracted (experience a lower effective nuclear charge). As Carbon's orbitals are higher in energy, they contribute more to the antibonding orbitals, hence why the π* lobe is much larger on carbon as highlighted in the LUMO diagram.
References
- ↑ http://cccbdb.nist.gov/exp2x.asp?casno=7664417
- ↑ http://ac.els-cdn.com/0021961472900390/1-s2.0-0021961472900390-main.pdf?_tid=ec5000e4-0586-11e7-a41d-00000aab0f6c&acdnat=1489146450_1367a44e2d4c0843e7ed654d41e9b959
- ↑ http://pubs.rsc.org/en/content/articlepdf/2003/nj/b301947k
- ↑ http://web.chem.ucla.edu/~harding/notes/notes_14D_C=Ofun02.pdf