Rep:Mod:mcpdpart2
Analysis of the properties of the synthesised alkene epoxides
The two catalytic systems
The crystal structure of the two catalysts
| The Shi asymmetric fructose catalyst | |||
|---|---|---|---|
|
The calculated NMR properties of 2-Phenyloxirane and trans-2,3-diphenyloxirane
Procedure
Each epoxide was drawn into ChemBio3D and saved in a .cml format after performing a MM2 force field optimization. The .cml files were opened in Avogadro and a MMFF94s force field minimization was performed. From the Extensions/Gaussian menu, the following parameters were implemented:
- Geometry optimisation (Opt + Freq)
- Theory: B3LYP
- Basis set: 6-31G(d,p)
- Solvation mode: CPCM, Chloroform
- Keywords: Freq NMR, Empirical Dispersion = GD3
The generated input file was submitted to the HPC system. When the job was indicated as finished, the formatted checkpoint file was downloaded and opened in Gaussview. The NMR spectra were accessed from Results/NMR, paying attention to select the corresponding basis set, the TMS reference and the chloroform solvent. The .txt files containing the chemical shifts were used for comparison with the literature. The spectra were also exported as .svg files. DOI:10042/25695
2-Phenyloxirane spectra
13C NMR
| δ Predicted (ppm) | δ Literature [1] (ppm) | Difference (ppm) |
|---|---|---|
| 135.1 | 137.5 | 2.4 |
| 124.1 | 128.4 | 4.3 |
| 123.4 | 128.1 | 4.7 |
| 122.9 | 125.4 | 2.5 |
| 118.3 | - | - |
| 54.0 | 52.2 | -1.8 |
| 53.5 | 51.1 | -2.4 |
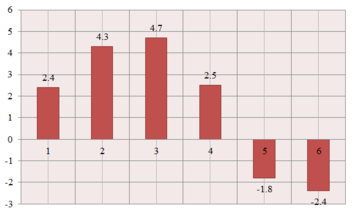
The molecule contains 8 carbon atoms (numbered structure). Though it is not symmetric (as can be seen in the 3D model, which shows C4-C6 and C1-C3 in slightly different environments compared to the epoxide structure), the literature assignment [1] recognizes only 6 carbon environments. This is most probably due to the rotation of the molecule and the resolution of the spectrometer (75 MHz, CDCl3); the C4-C6 and C1-C3 carbons are averaged and "seen" as equivalent.
The predicted chemical shifts are reasonably close to the experimental values (the differences are represented in the graph on the right). However, 7 carbon environments rather than 6 are expected to be seen in this case. According to the .txt output file, C2 and C6 are equivalent and there is an extra peak corresponding to C4. This result is quite surprising, because C2 and C6 are clearly in different environments; it would have been more understandable to have one of the C1-C3 or C4-C6 pairs seen as equivalent. It therefore follows that the degeneracy predicted by the calculation is accidental, and there should actually be 8 signals predicted, confirming the actual lack of symmetry of the structure.
A more accurate comparison of the predicted shifts with the experimental values involves averaging the chemical shifts for the C4-C6 and C1-C3 carbons to mimic the averaging observed in reality. The following correlation follows (1st number = predicted, 2nd number = literature; units = ppm):
- 135.1 -> 137.5; 120.55 -> 128.4; 123.75 -> 128.1; 122.9 -> 125.4; 54 -> 52.2; 53.5 -> 51.1.
While there are differences in the chemical shift values, the agreement with the literature is reasonable.
The spectrum clearly distinguishes between the aromatic region (135.1-118.3 ppm) and the aliphatic part corresponding to the epoxide part of the molecule (54.0-53.5 ppm).
- Note: The numbering of the carbon atoms was taken to be the same as in the .cml file loaded into Avogadro for producing the HPC input file; that .cml file was used to produce the jmol representation.
- The output spectrum can be accessed here.
1H NMR
| δ Predicted (ppm) | δ Literature [1] (ppm) |
|---|---|
| 7.51-7.29 (5 signals) | 7.33-7.28 (5H multiplet) |
| 3.66 | 3.86 |
| 3.12 | 3.15 |
| 2.54 | 2.81 |
The predicted 1H NMR spectrum is in good agreement with the literature[1] (300 MHz, CDCl3), with small differences between the predicted and actual chemical shift values. The spectrum is comprised of the aromatic region at ~7 ppm corresponding to the 5 aromatic protons and the aliphatic one with 3 epoxide signals at ~3 ppm (deshielded due to the oxygen atom proximity).
- The output spectrum can be accessed here.
2,3-diphenyloxirane spectra
13C NMR
| δ Predicted (ppm) | δ Literature [2] (ppm) | Difference (ppm) |
|---|---|---|
| 134.1 | 137.1 | 3 |
| 124.2 | 128.6 | 4.4 |
| 123.5 | 128.3 | 4.8 |
| 123.2 | 125.5 | 2.3 |
| 118.3 | - | - |
| 66.4 | 62.8 | -3.6 |

The molecule contains 14 carbon atoms (numbered structure). For symmetry reasons, only 5 chemical environments appear to be present, with the following groups of carbon atoms being equivalent: C7-C8, C4-C9, C3-C5-C10-C14, C2-C6-C11-C13, C1-C12. Indeed, there are 5 signals reported in the literature [2]. However, the prediction refers to 6 rather than 5 shifts, with an extra signal at 118.3 ppm. According to the NMR report, this signal corresponds to C5 and C14. Analysing the peak list it can be seen that the prediction takes the following couples of carbons as equivalent: C7-C8, C4-C9, C3-C10, C5-C14, C2-C11, C6-C13, C1-C12. This gives some insight into the extent of symmetry of the molecule in the sense that atom pairs such as C5 and C10 do not "see" the exact same environment, being at different difference compared to the epoxide part of the molecule. However, these subtle differences are not detected by the resolution of the spectrometer in the literature (125 MHz, CDCl3). It therefore follows that the prediction gives a more accurate insight into the symmetry of the molecule.
The predicted chemical shifts are reasonably close to the experimental values (the differences are represented in the graph on the right). The spectrum clearly distinguishes between the aromatic region (134.1-118.3 ppm) and the aliphatic part corresponding to the middle epoxide section (66.4 ppm).
- Note: The numbering of the carbon atoms was taken to be the same as in the .cml file loaded into Avogadro for producing the HPC input file; that .cml file was used to produce the jmol representation.
- The output spectrum can be accessed here.
1H NMR
| δ Predicted (ppm) | δ Literature [2] (ppm) |
|---|---|
| 7.57-7.47 (10 signals) | 7.40-7.32 (10H multiplet) |
| 3.54 | 3.87 |
The predicted 1H NMR spectrum is in good agreement with the literature [2](500 MHz, CDCl3), with small differences between the predicted and actual chemical shift values. The spectrum is comprised of the aromatic region at ~7.5 ppm corresponding to the 8 equivalent aromatic protons and the aliphatic one with an epoxide signal at 3.54 ppm corresponding to the two equivalent aliphatic protons (deshielded due to the oxygen atom proximity.
- The output spectrum can be accessed here.
Assigning the absolute configuration of the product
The reported literature for optical rotations (589 nm, CHCl3)
The values for the optical rotation were searched using Reaxys.
2-Phenyloxirane
(R) form: [α]20D = -19.5 deg[3]
(S) form: [α]20D = +20 deg[4]
2,3-diphenyloxirane
(2R, 3R) form: [α]22D = +250.8 deg[5]
(2S, 3S) form: [α]20D = -205.2 deg[2]
Optical rotation calculations
Procedure
The formatted checkpoint files for the previous NMR computations were downloaded and opened in Gaussview. They were saved as .gjf files, then opened in Notepad++ to implement the following parameters:
- cam-b3lyp/6-311++g(2df,p) polar(optrot) scrf(cpcm,solvent=chloroform) CPHF=RdFreq
- wavelengths: 589 nm 365 nm
The resulting log files were inspected to find the [alpha]D values for each molecule.
Results
2-Phenyloxirane (DOI:10042/25705 )
[α]D = - 30.36 deg. This is comparable to the literature [3] value of -19.5 deg, which suggests that the drawn isomer is (R). The closeness of the computed and actual values is accurate enough given the use of a non-tailored basis set.
The reliability of the computation was tested by drawing the other enantiomer (S) and computing, following the same procedure, its optical rotation. A value of +30.44 deg was obtained, which mirrors the -30.36 deg previously computed, validating the calculation. (DOI:10042/25715 )
2,3-diphenyloxirane (DOI:10042/25706 )
[α]D = + 299.42 deg. This is comparable to the literature [5] value of +250.8 deg, which suggests that the drawn isomer is (R,R). The closeness of the computed and actual values is accurate enough given the use of a non-tailored basis set.
The reliability of the computation was tested by drawing the other enantiomer (S, S) and computing, following the same procedure, its optical rotation. A value of - 297.98 deg was obtained, which mirrors the +299.98 deg previously computed, validating the calculation. (DOI:10042/25731 )
Electronic circular dichroism (ECD) spectrum calculation
- Note: Due to the absence of a chromophore, 2-Phenyloxirane was not modeled for this part. 2,3-diphenyloxirane does not seem to have a strong chromophore either, but was nevertheless attempted to be studied.
Procedure
The formatted checkpoint file for the previous NMR computation for 2,3-diphenyloxirane was downloaded and opened in Gaussview. It was saved as .gjf files, then opened in Notepad++ to invoke the following keywords:
- CAM-B3LYP/6-311+G(d,p) td(NStates=20) scrf(cpcm,solvent=chloroform)
The file was submitted on the HPC system and the output log file was opened in Gaussview. The predicted UV-Vis and ECD spectra were accessed from Results/UV-Vis. The axes were inverted to allow for literature comparisons.
ECD spectrum:

UV-Vis spectrum:

Discussion
As the above image shows, the maximum absorption in the ECD spectrum is between 200 and 220 nm, which is too low to be measured in practice. Therefore no comparison with the literature is possible and this calculation does not give any useful insight into the properties of the epoxide. If, however, the molecule also contained groups such as -NO2, a chromophore would have definitely been present and a comparison with the literature would have probably been possible.
Predicting the stereochemical outcome of the β-methyl styrene epoxidation
Shi epoxidation of trans β-methyl styrene
The transition states for the R,R and S,S series for the Shi epoxidation of trans β-methyl styrene available in the script were inspected and the minimum free energy values for each series were selected after accessing the log files linked in the tables (the energies provided by the log files are corrected for entropy, zero-point thermal energies and solvation).
To work out the enantiomeric excess, the following transformation was imagined:
(R,R) TS -> (S,S) TS
The energy difference between the two states was then converted into units of kJ mol-1. To work out the equilibrium constant, the following equation was used: ΔG = -RT ln K (therefore K = exp (ΔG/(-RT)), R = 0.008314 kJ mol-1, T = 298.15 K). The table below summarizes the results of these calculations:
| R,R series min (Hartree) | S,S series min(Hartree) | Difference (Hatree) | Difference (kJ mol-1) | Equilibrium constant (K) |
|---|---|---|---|---|
| -1343.03244 | -1343.024742 | 0.007701 | 20.2189755 | 0.000287 |
| R,R |
S,S | |
|---|---|---|
| Initial | 1 | 0 |
| Consumed | x | 0 |
| Final | 1-x | x |
Using the rationale on the left, the equilibrium constant is equal to x/1-x. Simple maths reveal that the value of x is 0.000286918, which make the final concentration of (R,R) 0.999713082 and that of (S,S), 0.000286918. Therefore the enantiomeric excess of (R,R) is over 99.97%, which means that this product is enantioselectively produced in the reaction. It was actually obvious from the very low value of K that the "reaction" imagined here is driven to the left, hence towards the (R,R) form.
In conclusion, the (R,R) enantiomer is expected to be enantioselectively produced in the epoxidation of trans β-methyl styrene using the Shi catalyst. This theoretical prediction is in agreement with the literature [6], where it is shown that the (R,R) form was obtained in 90% ee.
Jacobsen epoxidation of cis β-methyl styrene
Similar to the previous computation for the epoxidation of the trans isomer, the transition states for the S,R and R,S series for the Jacobsen epoxidation of cis β-methyl styrene available in the script were inspected and the minimum free energy values for each series were selected after accessing the log files linked in the tables. The free energy difference was found in the same way as before, imagining the transformation (S,R) TS -> (R,S) TS.
The following table shows the values that were used in the calculation:
| S,R series min (Hartree) | R,S series min(Hartree) | Difference (Hatree) | Difference (kJ mol-1) | Equilibrium constant (K) |
|---|---|---|---|---|
| -3383.259559 | -3383.25106 | 0.008499 | 22.3141245 | 0.000123 |
Following the same calculation procedure as before, the final concentration of (S,R) is found 0.999877015 and that of (R,S), 0.000122985. Therefore the enantiomeric excess of (S,R) is over 99.98%, which means that this product is enantioselectively produced in the reaction. Again, this is also obvious from the very low K value found above, which favours the left side of the reaction.
In conclusion, the (S,R) enantiomer is expected to be enantioselectively produced in the epoxidation of cis β-methyl styrene using the Jacobsen catalyst. This theoretical prediction is in agreement with the literature [7], where it is shown that the (S,R) form was obtained in 92% ee.
Investigating the non-covalent interactions in the active-site of a reaction transition state ( β-methyl styrene epoxidation)
Procedure
The .fchk file of the first transition state in the R,R series for the Shi epoxidation of β-methyl styrene (DOI:10.6084/m9.figshare.738028 ) was downloaded and opened in Gaussview.
- Results - Surfaces - Contour - Cube Actions - New Cube.
- Options: Total density, Grid = Medium.
- When the cube was shown available, it was saved into the temp directory on the C drive.
- This pagewas accessed to convert the density cube into an NCI surface.
- The .xyz and .jvxl files generated were saved.
Results
| NCI surface | |||
|---|---|---|---|
|
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
Procedure
Suggesting new candidates for investigations
Procedure
Reaxys was searched for epoxides (as a substructure). The following advanced property was implemented: ORP.ORP<'-500' or ORP.ORP>'500'. The query returned 123 hits.
Proposal
References
- ↑ 1.0 1.1 1.2 1.3 D. Forbes, S. Bettigeri, S. Patrawala, S. Pischek, M. Standen, Tetrahedron, 2009, 65, 70–76. DOI:10.1016/j.tet.2008.10.019 Cite error: Invalid
<ref>tag; name "cnmrstyr" defined multiple times with different content - ↑ 2.0 2.1 2.2 2.3 2.4 T. Niwa, M. Nakada, J. Am. Chem. Soc, 2012, 134 , 13538–13541. DOI:10.1021/ja304219s
- ↑ 3.0 3.1 J. Schrittwieser, W. Kroutil, I. Lavandera, B. Seisser, B. Mautner, J. Lutje Spelberg, Tetrahedron: Asymmetry, 2009, 20 , 483 - 488. DOI:10.1016/j.tetasy.2009.02.035
- ↑ A. Piccinini, S. Kavanagh, S. Connon, Chemical Communications (Cambridge, United Kingdom), 2012, 48 , 7814 - 7816. DOI:10.1039/C2CC32101G
- ↑ 5.0 5.1 D. Fox, D.S. Pedersen, S. Warren, Org. Biomol. Chem., 2006, 4 , 3117-3119. DOI:10.1039/b606881b
- ↑ A. Wong, B. Wang, M-X Zhao, Y. Shi,J. Org. Chem., 2009, 74 , 335–6338. DOI:10.1021/jo900739q
- ↑ E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker, L. Deng, J. Am. Chem. Soc., 1991, 113, 7063–7064. DOI:10.1021/ja00018a068
