Rep:Mod:maxk1
Module 1
Part 1
Cyclopentadiene
Cyclopentadiene dimer 1
Maxkindred1.1.1 |
Torsion: 7.6718
Total Energy: 31.8834 kcal/mol
Cyclopentadiene dimer 2
Maxkindred1.1.2 |
Torsion: 9.5157
Total Energy: 34.0071 kcal/mol
The relative torsion strain and total energies of thesee two molecules awould suggest that the first dimer is the more thermodynamically stable product. This would suggest that the dimer created in the synthesis, was created under kinetic conditions as is quite often the case with diels-alder reactions. The kinetic form is the one created due the stability of the transition state, as the kinetic t.s. is the less sterically hindered form.
Cyclopentadiene dimer 3
Maxkindred1.1.3 |
Stretch: 1.2382
Bend: 18.9595
Torsion: 12.1018
Non-1,4 VDW: -1.4892
1,4 VDW: 5.7264
Total Energy: 35.6908 kcal/mol
Cyclopentadiene dimer 4
1.1.4 |
Stretch: 1.0896
Bend: 14.5179
Torsion: 12.5142
Non-1,4 VDW: -1.0490
1,4 VDW: 4.5010
Total Energy: 31.1718 kcal/mol
The overall energy of the 2 isomers suable product suggests that the dihydro derivative 4 is the more thermodynamically stable product as the double bond on the bridged 5 membered ring is more sterically hindered and therefore atttack here gives a more stable product. This is backed up by the other relative properties, the relative stretch, bend and VDW energies are significantly larger for the less stable derivative, dimer 3. The torsion values are very similar for both and the H-bonding is negligible as there are no electronegative atoms with lone pairs for any Hydrogen bonding to occur.
Nucleophilic addition to a Pyridinum Ring
If the MeMgI is inlcuded in the energy minimization for molecule 5 chem bio 3d produces an error file because the program does not understand bonding involving Mg atoms.
Molecule 5
Max1.1.5 |
Stretch: 2.2344
Bend: 14.1252
Stretch-Bend: 0.1325
Torsion: 5.0714
Non-1,4 VDW: -0.5186
1,4 VDW: 16.5496
Total Energy: 48.1377 kcal/mol
As can be seen from the rotating Jmol the carbonyl Oxygen is slightly above (8.5 degrees) the plane of the pyridinium ring and thefore when the incoming Organometallic reactant chelates to this carbonyl group it directs the methyl substituent onto the same face as the oxygen (the top face) at the 4 postion via the mechanism below.

Max1.1.5wrong |
Stretch: 3.8490
Bend: 112.1884
Stretch-Bend: -0.2596
Torsion: 27.6170
Non-1,4 VDW: 0.9602
1,4 VDW: 29.8703
Charge/Dipole: -12.9192
Dipole/Dipole: -3.5802
Total Energy: 157.7258 kcal/mol
This is the only other place where the carbonyl will not revert to the position in the first example upon energy minimization but this conformation immediately sets off alarm bells due to the angles present on the bonds in the carbonyl group. This molecule can easily be drawn in 2d but upon minimization the energy and the bend values are extremely high so this molecule is unrealistic.
Molecule 6
Stretch: 2.2405
Bend: 16.2891
Stretch-Bend: 0.3305
Torsion: 5.6543
Non-1,4 VDW: -0.1921
1,4 VDW: 17.4977
Total Energy: 37.4646 kcal/mol
Molecule 7
1.1.7 |
Stretch: 4.0850
Bend: 12.8146
Stretch-Bend: 0.4547
Torsion: 12.1794
Non-1,4 VDW: 4.7597
1,4 VDW: 29.1029
Total Energy: 72.1348 kcal/mol
In this molecule the carbonyl group is 25 degrees above the plain of the ring, directing the incoming reactant to the top face of the molecule (machanism)
Taxol
Molecule 10
Max1.1.10 |
Stretch: 2.4608
Bend: 10.8267
Stretch-Bend: 0.3010
Torsion: 17.3617
Non-1,4 VDW: -1.5920
1,4 VDW: 12.3564
Dipole/Dipole: 0.1438
Total Energy: 41.8583 kcal/mol
Molecule 11
Max1.1.11 |
Stretch: 2.5628
Bend: 10.7438
Stretch-Bend: 0.3335
Torsion: 19.7397
Non-1,4 VDW: -1.4427
1,4 VDW: 12.5436
Dipole/Dipole: -0.1785
Total Energy: 44.3022 kcal/mol
As can be seen from relative energies of these two molecules, molecule 10 is the more stable isomer, with the carbonyl group pointing up. This isomer has much less steric hinderance sorrounding the oxygen from the cyclohexane ring and the pentane ring.
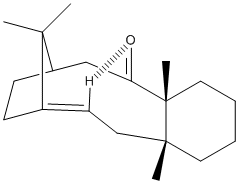
The above does however mean that the carbonyl is further away from the double bond so may reduce the hinderance to attack from electrophiles, also when the carbonyl group is up there is the possibility of H-bonding across the molecule to the Hydrogen on the double bond making that double bond less reactive, see diagram. Due to these ovbservations the double bond on molecule 11 is thought would be more reactive.
The double-bond in large ringed structures like taxol can be "hyperstable" as the double bond has a bond angle of 120 degrees compared to 109 for a saturated carbon which allows the molecule to adopt a conformation for which if it were a single bond would have too high strain. <2>
Both these molecules above contained the cyclohexane ring in the chair formation, the twist-boat formation was also isolated for both, molecule 10 (twist-boat) had Total Energy: 54.1108 kcal/mol, molecule 11 (twist-boat) had Total Energy: 48.1586 kcal/mol. Neither boat form could be isolated.


Modelling Using Semi-empirical Molecular Orbital Theory.
The molecule was found to have two flat rings and and the Cl above one double bond and its opposite hydrogen above the other. The homo of molecule 12 does diferentiate between the 2 double bonds, showing electron density on the Carbon-carbon double bond syn to he Cl molecule meaning that this bond would be more susceptible to any electrophilic attack on the molecule making that doube bond more nucleophilic.
The diene and the alkene derivative both only have one major C-Cl stretch, which occur at 770.89 and 775.15 cm-1 and have an intensity of 25.1 and 19.9 respectively. The major C=C stretch for the mono alkene derivative comes at 1758cm-1 and for the diene at Normal 0 false false false EN-GB X-NONE X-NONE 1737.13, 1757.37cm-1 with similar frequencies.
References
P. Camps, X. Pujol, S Vazquez, M. A. Pericas, C Puigjaner and L. Sola, Tetrahedron, 2001, 57, 8511
Module 2
the energy will have an error of ≈ 10 kJ/mol, so how much is this in atomic units (which are hartree)? the dipole moment will be accurate to about 2 decimal places, ie 0.01 Deby. frequencies in wavenumbers are by convention reported with no decimal places and for the purposes of this course you need to know that the accuracy is only to around 10cm-1 (the situtation is actually more complex, and is covered in my fourth year course "Computational Inorganic Chemistry") intensities are rounded to the nearest whole integer and in fact the accuracy is much less that this, but this is convention bond distances are accurate to ≈ 0.01 Å bond angles are accurate to ≈ 0.1
BH3
The bond lengths for BH3 are all equal after optimisation at 1.19A and all the HBH angles being 120 degrees. The NBO analysis shows each Hydrogen carrying -0.111 charge each and the Boron being +0.332 showing that the boron is electropositive as has been shown by its chemical (lewis acidic) properties.
BCl3
THe method used to optimise the BCl3 molecule was DFT and the basis set used was LANL2MB
Both calculations??
The reason we have to carry out a frequency analysis of the molecule is to make sure that the position of zero gradient found by the optimisation is in fact the minimum energy rather than the maximum energy. The analysis carries out a second derivative calculation to determine this.
The BCl3 bond length is 1.87A and the bond angle is 120 degrees.
Gaussview does not draw certain bonds in certain molecules as gaussview has been programmed to recognise bonds only up to a certain length, which is roughly around the size of a C-C bond, anything significantly longer than that e.g Metal – Phosphorus bonds , does not compute as a bond. This however does not represent a huge problem as the presence of a bond is for our purpose only and does not affect any calculations if it is there or not.
A bond is a region where there is a favourable overlap between 2 or more aligned orbitals (whether they be s, p or d) on adjacent molecules. The orbitals must be populated and the population of these orbital must lower the overall energy of the molecule for the bond to form.
The symmetry you expect to see in the ground state configuration is d3h, the same as the one you see from your Gaussian optimisation. This is interesting, as you would expect upon optimisation, the symmetry of the molecule to change to a trigonal pyramidal molecular geometry and tetrahedral electronic geometry with the B empty p orbital going up above the molecule. This form is lower in energy than the trigonal planar molecule. Gaussian does not allow the molecule to adopt this formation as it doesn’t allow itself to break the symmetry of the molecule.
All these calculations are rather simple and can be conducted on the Chemistry Laptops and each take less than a minute.
Below is a MO diagram for BH3, containing the LCAO's made up of combined atomic orbitals, and the orbitals created by Gaussian. The orbitals created by Gaussian look slightly different to the LCAO's, the nodes are still in the same place but all positive overlap appears as one block of electron density.

Isomers of Mo(CO)4L2
| Lit vs model | Lit | Model(Trans)/A | Model(Cis)/A ! | |
|---|---|---|---|---|
| C=O | 1.18 | 1.19 | 1.19 | |
| Mo-C | 2.08 | 2.1 | 2.1 | |
| Mo-P | 2.4 | 2.5 | ||
| P-Cl | 2.04 (non-metal bonded | 2.4 | 2.4 |
The cis isomer is slightly higher in energy than the trans one (although its probably outside the accuracy of this method) due to the extra steric strain caused by the proximity of the larger PCl3 groups to each other. The E difference is .003 a.u .
Changing the size of the R groups on the PR groups will change the ratio of cis/trans isomer found as the reduction/increase of sterics in the cis form, e.g. changing R group to Ph would greatly increase the energy of the cis isomer as Ph is a much larger group than Cl.
There are no vibrations of a very low energy in the trans isomer, the cis isomer has 2 low energy vibrations at -2 and 12, when a vibration is this low in energy , this means there is a very low energy vibration for this vibration to occur. Therefore at room temperature this vibration will occur very rapidly.
Carbonyl stretches (cis) cm-1
Intensity
1808.87
1306.71
Unsymetric
1809.01
1297.17
Unsymetric (opposite to 1808.87)
1831.78
1.99
Symmettric
1881.57
0.06
Totally symmetric
This table shows that in reality there are only 2 viable unsymmetrical carbonyl stretches, but the fact the other two vibrations are present suggest that the molecule is not completely symmetrical because if it was these vibration would have an intensity of 0.
Carbonyl stretches (trans) cm-1
Intensity
1805.17
1161.96
1806.21
748.16
1815.05
621.53
1872.85
695.53
All these vibrations change the overall dipole moment of the molecule and therefore all have large intensities, these are the number of C=O shifts we would expect to see for each molecule, due to their different symmetries.
MiniProject
For my mini-project i have chosen to study the catalytic cycle of hydroformylation, in particular using the starting catalyst, HRh(Co)(PPh)3. However in order to speed up calculations Cl was used in place of the Ph groups.
The cycle would be studied in 3 different places marked 1, 2 and 3 on the picture below, representing the start of the cycle, where the substrate adds on and where the substrate is eliminated. The Mo’s for these three intermediates will be studied as will there charge distribution and CO vibrational frequencies to see how these change through the cycle.
The series will them be expanded to take in a series of metals from the same group as Rh and the charges, bond lengths and relative energies compared with that of the Rh complexes in order to try to ascertain why Rh is the best metal for this catalytic cycle.
The MO for molecule 1
The homo shows a bonding interaction between the Rh and the CO, a non-bonding interaction between the Rh and the H and a anti-bonding interaction between the Rh and the PCl3 groups.
The lumo shows similar carachteristics, from this it can be shown that upon substitution reactions, if an associative mechanism were used, if the Lumo was filled there would be a weakening of the PCl3 bonds and a strengthening of the CO bonds. If a dissociative mechanism were used the PCl3 has the weakest interaction at HOMO level so would be the most likely to leave. This explains why it is a PCl3 group that leaves when the alkene substitutes in rather than a CO group.
The homo-1 shows a bonding interaction between the metal and one P group and a small bonding interaction with both CO groups.
The homo-2 shows a strong bonding interaction with the CO groups and non-bonding interactions with the PCl3 groups.
The lumo +1 shows a bonding interaction between the metal and the Co and Pcl3 groups but a nonbonding interation between the metal and the H aswell as non-bonding interactions between the C and the O and the P and the Cl
The lumo +2 is non-bonding with respects to the metal and will therefore not be looked at in detail.
The IR spectras shows 2 CO stretch vibrations at
