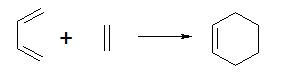Rep:Mod:m190l127
The Diels Alder Cycloaddition
Reaction: Cis-butadiene + Ethylene
In order to understand the stereospesific nature of this reaction it is vital to know the interaction of π bonds of two reaction fragments invovled in this reaction. first the frontier orbital of one of the reactants, cis-butadiene.
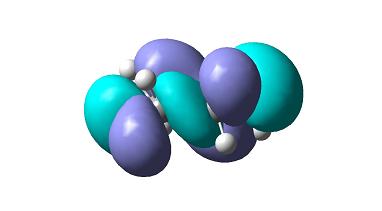
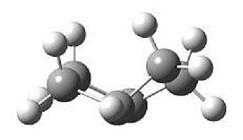
Now consider transition state of this reaction, which looks like the diagram and can be considered as a di-cyclo system system structure. the double bond of the cis-butadiene is located along the two carbons on the left.
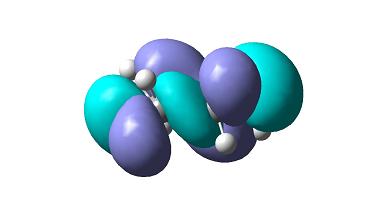
Frontier orbitals of this transition state are as follows.
as can be seen HOMO molecular orbital is symmetric whereas LUMO molecular orbital isnt.
Reaction:Cyclohexa-1,3-diene with Maleic Anhydride
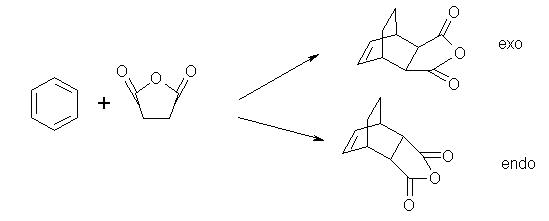
The energies of two end products are -462.60618581 a.u and -462.61035741 a.u respectably. Since diels alder is a kinetically controlled reaction so exo product is supposed to have higher energy as can be seen.