Rep:Mod:ly2112
Abstract
The Gaussian 09W and Gaussview 5.0 was used to modified and calculate the transition state of reactions involves Diels-Alder cycloaddition and cheletropic addition. The MO diagram and the activation energies were presented and analysed for different geometry of products and to obtained a preferred path way for reactions.
Nf710 (talk) 16:14, 24 January 2017 (UTC) You should have defined a TS here with second derivatives etc.
Reaction of Butadiene with Ethylene

The reaction of cis-butadiene with ethylene is a Diels-Alder reaction as a [4+2] cycloaddition reaction. The [4+2] indicates the number of electrons involved in this reaction. A six-membered ring is formed by converting two bond to Two bonds during the reaction.
Optimise the reactants and TS
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | Point Group | HOMO | LUMO |
|---|---|---|---|---|---|
| Butadiene (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:2BUTENE.LOG) | 0.09923 | C2 | 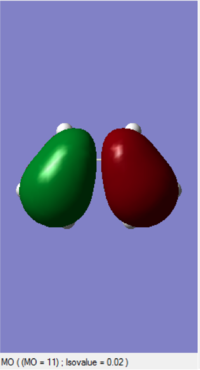
|
| |
| Ethylene (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:1ETHENE.LOG) | 0.049447 | C2 | 
|
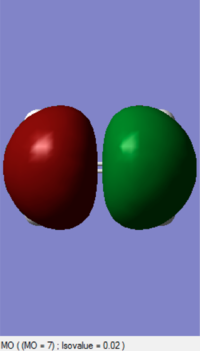
|
| |
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
| Cyclo TS (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:5UDKS_5.LOG) | 
|
0.2126 | 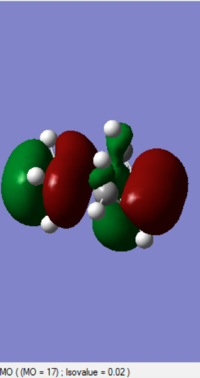
|
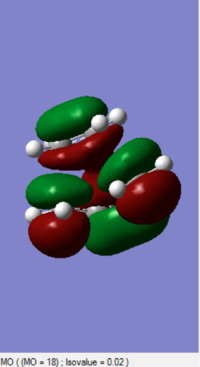
|
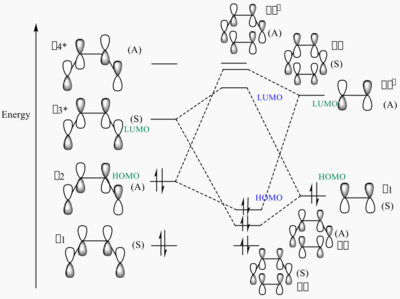
MO diagram for the formation of the butadiene/ethene TS
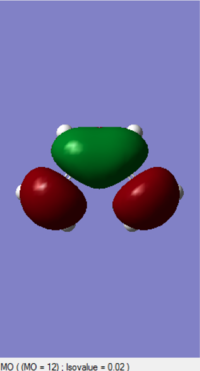
The MOs of HOMO and LUMO is shown. The allowed reaction must be followed the rule that the symmetries of the reactants and the transition state must be consistent. By analysing and comparing the MO diagraph of reaction and visualised the MO form the chk. File, the corresponding MO has proved the theory. The symmetric HOMO of ethylene reacts with the symmetric LUMO of butadiene to form the symmetric LUMO of transition state, and the asymmetric LUMO of ethylene reacts with the asymmetric HOMO of butadiene to form the asymmetric HOMO of transition state.
Nf710 (talk) 16:20, 24 January 2017 (UTC) I am really struggling to see what you are trying to show with the second MO diagram.
The orbital interaction

The symmetric-symmetric interaction and asymmetric-asymmetric interaction has non-zero overlap integral as their orbitals overlap.
Optimise the final product
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
| Final Product (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:3FINAL_PRODUCT_CYCLOADDTION.LOG) | 
|
0.105183 | 
|
|
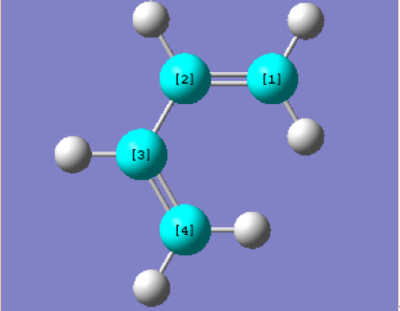
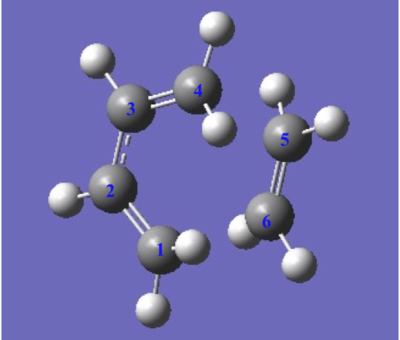
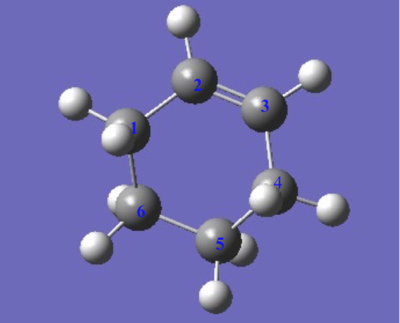
C-C bond length of the TS and product
 |
 |
|
The C,C bond (C1-C2, C3-C4) of butadiene in transition state(TS) is larger than it original value which is larger than the C=C bond and shorter than C-C bond. On the other hand, the C2-C3 bond of butadiene in TS is shorter than the C-C single bond of butadiene. Therefore the appearance of the bond length in transition state gives the evidence of the resonance of electron across the whole structure, and the C5-C6, C6-C1 bond length which is shorter than van Der Waals’ radius and longer than the typical SP3 C-C bond length indicates that the formation of a new C-C single bond between these two reactants. The final product showed a C-C bond length close to the typical SP3 C-C bond, but the two C-C bond(C1-C2), (C3-C4)which close to the double is slightly smaller than the literature value.
| Bond Length/Å | C1-C2 | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C1 | Typical SP2 C=C Bond | Typical SP3 C-C Bond | Van Der Waals Radius of C,C |
|---|---|---|---|---|---|---|---|---|---|
| Butadiene | 1.335 | 1.468 | 1.335 | 1.34 | 1.54 | 1.70 | |||
| Ethylene | 1.327 | ||||||||
| TS | 1.395 | 1.395 | 1.395 | 2.200 | 1.395 | 1.908 | |||
| Final Product | 1.500 | 1.338 | 1.500 | 1.540 | 1.541 | 1.540 |
vibration at the transition state
| Frequency/cm-1 | asynchronous | ||
|---|---|---|---|
| -948.34 |
| Frequency/cm-1 | asynchronous | ||
|---|---|---|---|
| 145.04 |
Nf710 (talk) 16:26, 24 January 2017 (UTC) This was a good first section, everything was done to a level of understanding, however you could have shown some understanding about normal or inverse demand. and I am still confused with what you were trying to show in the second MO diagram.
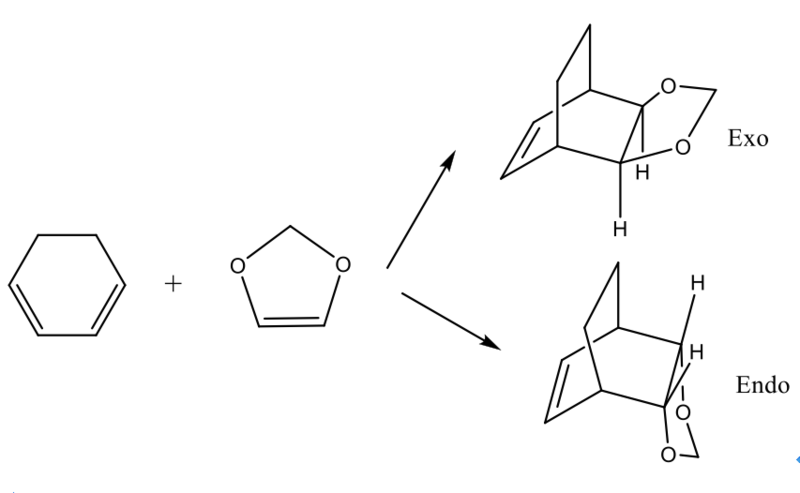
Reaction of Cyclohexadiene and 1,3-Dioxole
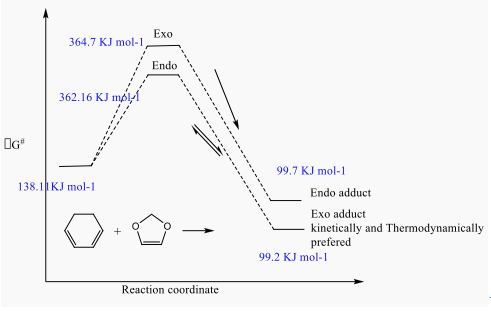
This reaction is a Diels Alder reaction with regio-selectivity. The transition state is optimised on both Semi-empirical/PM6 level and B3LYP/6-31G(d). Both reactants are optimised first on Semi-empirical/PM6 level to get a better geometry, followed by freezing the bond distances between reactive points of both reactants. 1,3-Dioxole is an electron poor dienophile due to the presence of two electrons withdrawing Oxygen therefore lower energy level for this reactant. In this case, the reaction is possibly to happened between the LUMO of 1,3-Dioxole and the HOMO of the Cyclohexadiene to undergo the normal Diels-Alder reaction. There are two conformation for the products, which are endo and exo form.
(Considering your introduction contradicts the conclusion of this section, you should start with something like "I would expect....", "however it was found that...". Recall that the methoxy group is electron donating Tam10 (talk) 12:10, 5 January 2017 (UTC))
Optimise the reactants
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
| Cyclohexadiene (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:CYCLODIENE_PM6_3.LOG) | 
|
0.118064 | 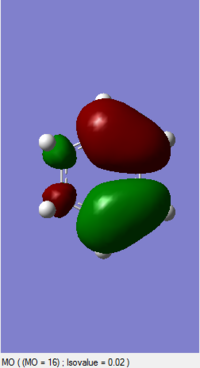
|
|
(Why are your orbitals PM6?
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
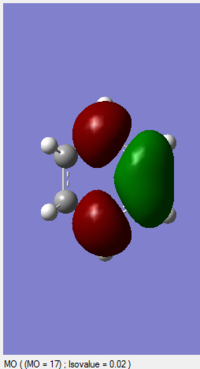
| 1,3-Dioxole (https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:2DIO_ENE_NEWEST.LOG) | 
|
-0.052279 | 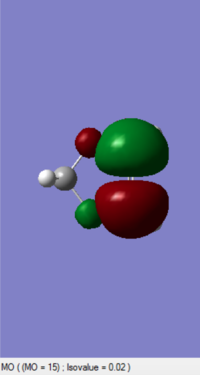
|
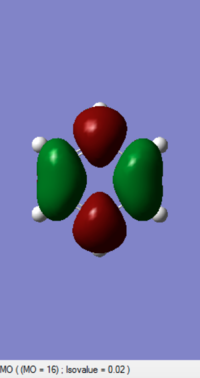
|
(The LUMO is not of 1,3-dioxole, and your HOMO is actually the LUMO. Tam10 (talk) 12:10, 5 January 2017 (UTC))
Optimise the ENDO form
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
| Endo(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:2016-12-16_10.48.49.png)(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:ENDO_TS_B3LYP631G_D.LOG) | 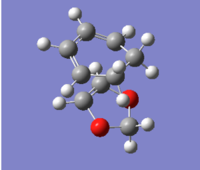
|
0.137941 | 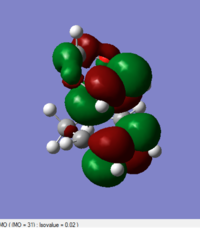
|
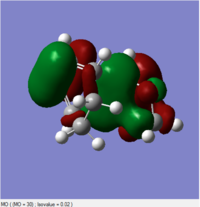
|
(Again, HOMO and LUMO are the wrong way around. This is also the exo TS Tam10 (talk) 12:10, 5 January 2017 (UTC))
Optimise the EXO form
| Molecule | Structure | Semi-Emirical Energy (Hartrees) | HOMO | LUMO |
|---|---|---|---|---|
| Exo(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:2016-12-16_10.55.07.png) | 
|
0.138906 | 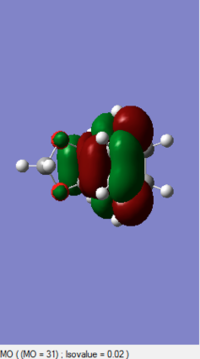
|
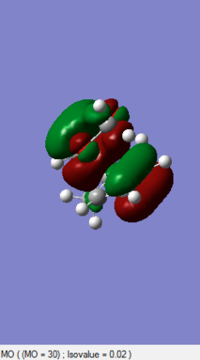
|
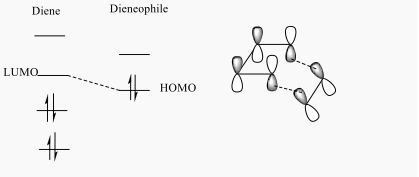
By comprising the MO diagrams of both TS, the reaction is happened between the LUMO of Cyclohexadiene and the HOMO of the 1,3-Dioxole to undergo the Inverse Diels-Alder reaction. This result indicates 1,3-Dioxole is an electron rich dienophile since it performs an inverse Diels-Alder reaction. The C=C bond of 1,3-Dioxole is possibly benefited from the donating of electron cloud density of Oxygen. This electro-donating effect raise the energy level of 1,3-Dioxole so the HOMO orbital of it is more close to the LUMO orbital of Diene.
(This does not correspond to what you've given for the endo though Tam10 (talk) 12:10, 5 January 2017 (UTC))
Compare The reaction path of two products
(Again, be careful. You've swapped endo and exo in the products Tam10 (talk) 12:10, 5 January 2017 (UTC))
Secondary orbital interaction
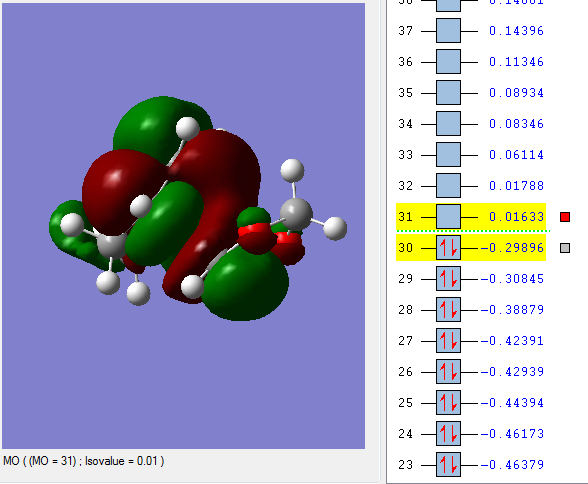
(This is the LUMO, which is unoccupied. The problem is you've used PM6, which happens to not show secondary orbital interaction Tam10 (talk) 12:10, 5 January 2017 (UTC))
The p-orbitals of Oxygen in endo conformation react with C=C bond and stabilise the transition state.
Therefore, there is a secondary reaction at HOMO for the endo form which lowered the reaction barrier, and stabilised the TS as well.
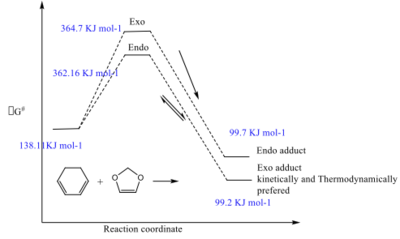
Endo has smaller activation energy and it is kinetically controlled, and less thermodynamically stable Exo has slightly higher activation energy but more thermodynamically stable. Overall, Exo conformation is preferred for this reaction.
The bonding of the 6-membered ring could be distorted during the reaction.
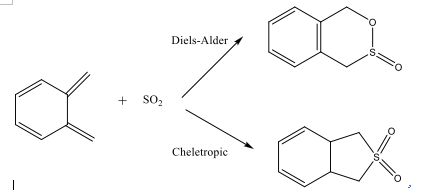
Diels-Alder vs Cheletropic
Optimise the TSs for the endo- and exo- Diels-Alder reaction
| Molecule | Structure | HOMO | LUMO |
|---|---|---|---|
| XYLYLENE DIELS ALDER ENDO TS PM6 1(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:XYLYLENE_DIELS_ALDER_ENDO_TS_PM6_1.LOG) | 
|
|
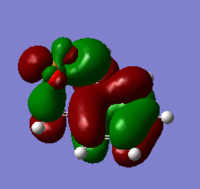
|
| Molecule | Structure | HOMO | LUMO |
|---|---|---|---|
| XYLYLENE DIELS ALDER TS PM6 1(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:XYLYLENE_DIELS_ALDER_TS_PM6_1.LOG) | 
|
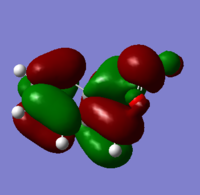
|
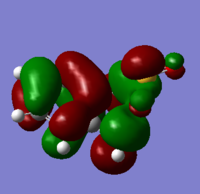
|
Optimise the TSs for the heletropic reactions
| Molecule | Structure | HOMO | LUMO |
|---|---|---|---|
| REACTANTS(https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:REACTANTS_NEW.LOG) | 
|

|

|
(This isn't the right TS, and the MOs are of cyclohexadiene Tam10 (talk) 12:10, 5 January 2017 (UTC))
IRC calculation for each path
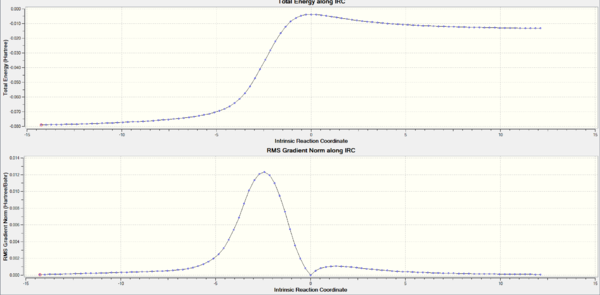
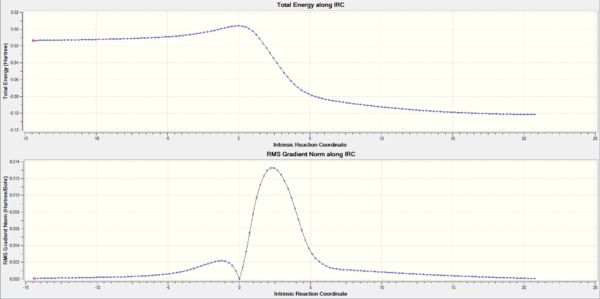
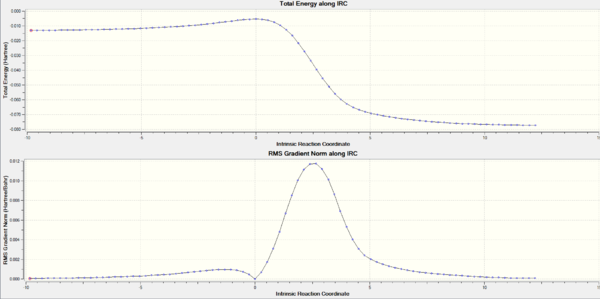
The activation and reaction energies for each step
relative heights of the energy levels of the reactants
Reference
1. Clayden, J., Greeves, N. and Warren, S. (2012). Organic chemistry. 2nd ed. Oxford: Oxford University Press.PP,P145