Rep:Mod:lxr1998
BH3 Section
BH3 optimisation
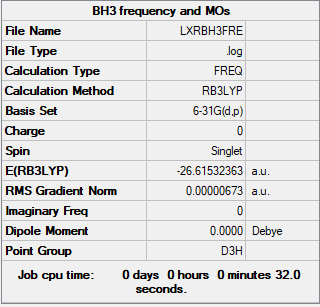
| Molecular Name | BH3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| B-H bond length | 1.192 Å |
| H-B-H bond angle | 120.00 |
| E(RB3LYP) | -26.61532 a.u. |
| Point Group | D3h |
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000042 0.001800 YES
RMS Displacement 0.000027 0.001200 YES
Predicted change in Energy=-6.625456D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1923 -DE/DX = 0.0 !
! R2 R(1,3) 1.1923 -DE/DX = 0.0 !
! R3 R(1,4) 1.1923 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Vibration Analysis
Low frequencies --- -7.5583 -1.5535 -0.0054 0.6544 6.9706 7.1416
Low frequencies --- 1162.9679 1213.1635 1213.1662
Diagonal vibrational polarizability:
0.7180491 0.7179489 1.8415328
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2" E' E'
Frequencies -- 1162.9679 1213.1635 1213.1662
Red. masses -- 1.2531 1.1072 1.1072
Frc consts -- 0.9986 0.9601 0.9601
IR Inten -- 92.5511 14.0538 14.0574
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.16 0.00 0.10 0.00 -0.10 0.00 0.00
2 1 0.00 0.00 -0.57 0.00 0.08 0.00 0.81 0.00 0.00
3 1 0.00 0.00 -0.57 -0.39 -0.59 0.00 0.14 0.39 0.00
4 1 0.00 0.00 -0.57 0.39 -0.59 0.00 0.14 -0.39 0.00
BH3 molecule |
The frequency file is liked to here
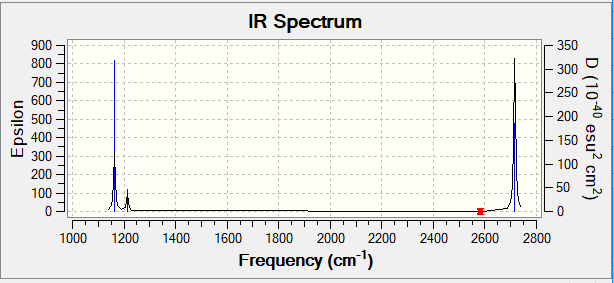
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A1 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
The only three peaks from the spectrum indicates there are three modes of vibration that can cause the change in the dipole moment. They are: out-of-plane bend and two modes of asymmetric stretch. The other three modes are not IR active because the vibrations do not change the moment dipoles.
Correct structure calculated and good consideration to the accuracy of the numbers when reporting the values in the table. It looks though like the table was copied and not changed fully from the NH3 example as the symmetries are incorrect (compare to the three caught by the screenshot above). Additionally, while your vibrational symmetry explanation is correct, it is correct for only one of the modes which are not visible and you should reconsider why the other two wouldn't appear. Smf115 (talk) 22:18, 15 May 2019 (BST)
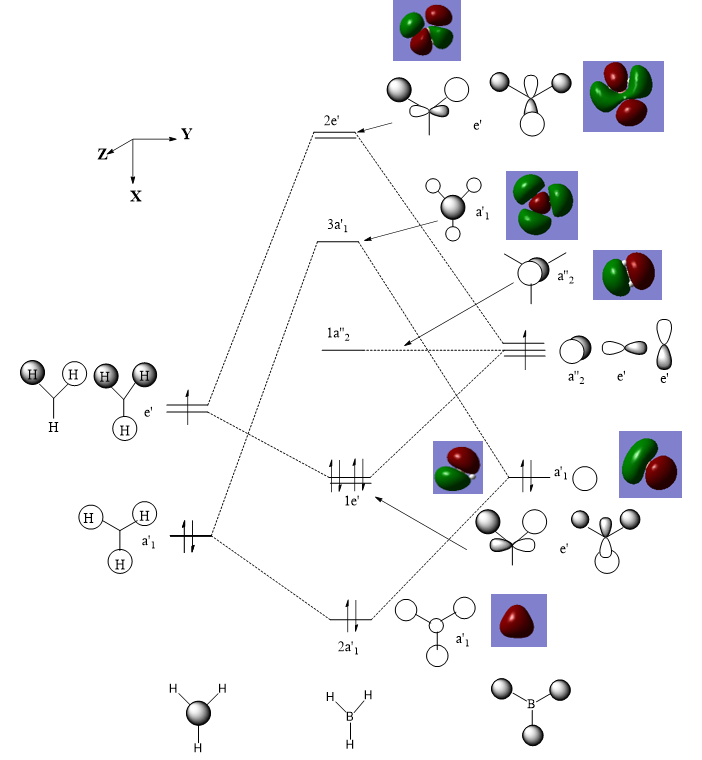
MO Diagram
There is no significant difference between the real and LCAO MOs. The only difference is that the real orbitals combine to form more diffuse electron clouds. THerefore, the MO theory is a useful approach to study the molecules.
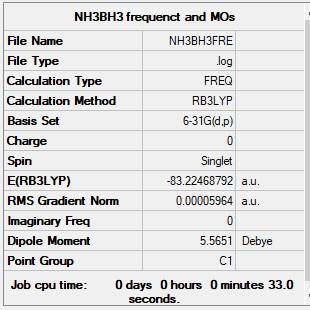
NH3 and NH3BH3 Section
Summary Tables
Low frequencies --- -426.8523 -426.8508 -385.8926 -0.0079 -0.0020 0.0076
Low frequencies --- 790.4012 1653.0881 1653.0884
Diagonal vibrational polarizability:
0.1933767 0.1933782 9.3178033
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A1 E E
Frequencies -- 790.4012 1652.8659 1652.8662
Red. masses -- 1.1863 1.0708 1.0708
Frc consts -- 0.4367 1.7235 1.7235
IR Inten -- 216.3092 19.3774 19.3776
Atom AN X Y Z X Y Z X Y Z
1 7 0.00 0.00 0.12 -0.07 0.00 0.00 0.00 0.07 0.00
2 1 0.00 -0.18 -0.54 0.77 0.00 0.00 0.00 0.13 0.23
3 1 0.16 0.09 -0.54 0.10 -0.39 0.20 0.39 -0.55 -0.11
4 1 -0.16 0.09 -0.54 0.10 0.39 -0.20 -0.39 -0.55 -0.11
Low frequencies --- -13.0650 -0.0018 -0.0012 -0.0009 19.1922 42.7349
Low frequencies --- 266.2925 632.1183 638.2195
Diagonal vibrational polarizability:
2.5469817 2.5597010 5.0173627
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 266.2917 632.1179 638.2193
Red. masses -- 1.0078 5.0027 1.0452
Frc consts -- 0.0421 1.1778 0.2508
IR Inten -- 0.0000 14.0632 3.5640
Atom AN X Y Z X Y Z X Y Z
1 1 0.45 0.03 0.00 0.00 -0.03 0.36 0.01 -0.21 0.57
2 1 -0.20 -0.40 -0.03 0.00 -0.03 0.37 -0.01 -0.18 -0.30
3 1 -0.25 0.37 0.03 0.00 -0.02 0.35 0.03 -0.18 -0.31
4 1 0.36 0.02 0.00 0.00 -0.01 -0.28 0.01 -0.14 0.45
5 1 -0.20 0.30 0.02 0.02 0.04 -0.30 0.03 -0.12 -0.24
6 1 -0.16 -0.33 -0.02 -0.03 0.03 -0.28 -0.01 -0.13 -0.23
7 7 0.00 0.00 0.00 0.00 -0.02 0.36 0.00 0.05 0.00
8 5 0.00 0.00 0.00 0.00 0.03 -0.48 0.00 0.03 0.00
You're missing the frequency log files for both calculations! Smf115 (talk) 22:25, 15 May 2019 (BST)
Association energy of NH3BH3 formation
| Energy of BH3 | -26.61532 a.u. |
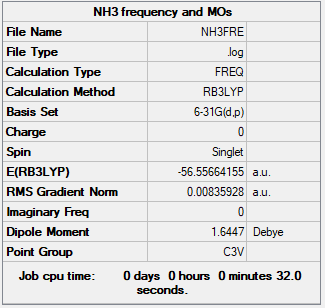
| Energy of NH3 | -56.55664 a.u. |
| Energy of NH3BH3 | -83.22469 a.u. |
| Association energy of formation | -0.05273 a.u. |
| Energy in kJ/mol | -138 kJ/mol |
The bond energy of the dative B-N bond, 138 kJ/mol, is medium compared to the strength of the normal C-C and B-H single bonds, which are around 346 and 389 kJ/mol respectively. This can be explained by the bonding is only contributed from two electrons from the nitrogen.
Nice and clear reporting of the energies of the structures. To improve you should have explicitly shown the calculation and your final answer is a little off, so seeing the calculation would help to identify where this has happened. Good comparisons used in the discussion but all literature values should be referenced! Smf115 (talk) 22:25, 15 May 2019 (BST)
NI3 Section
Item Value Threshold Converged?
Maximum Force 0.000102 0.000450 YES
RMS Force 0.000075 0.000300 YES
Maximum Displacement 0.000667 0.001800 YES
RMS Displacement 0.000490 0.001200 YES
Predicted change in Energy=-9.238359D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 2.1842 -DE/DX = -0.0001 !
! R2 R(1,3) 2.1842 -DE/DX = -0.0001 !
! R3 R(1,4) 2.1842 -DE/DX = -0.0001 !
! A1 A(2,1,3) 110.8579 -DE/DX = 0.0 !
! A2 A(2,1,4) 110.8579 -DE/DX = -0.0001 !
! A3 A(3,1,4) 110.8579 -DE/DX = -0.0001 !
! D1 D(2,1,4,3) 123.5674 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
Low frequencies --- -22.6972 -22.6939 -19.8471 -0.0037 0.0042 0.0248 Low frequencies --- 101.6652 101.6655 153.2725
NI3 molecule |
The frequency file is liked to here
The N-I bond length = 2.184 Å.
Project Section: Ionic Liquid
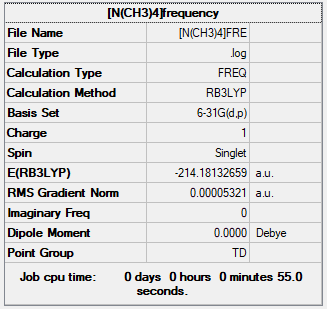
Optimisation and frequency analysis of [N(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000072 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001229 0.001800 YES RMS Displacement 0.000387 0.001200 YES Predicted change in Energy=-6.447467D-08 Optimization completed. -- Stationary point found.
Low frequencies --- -0.0013 -0.0010 -0.0004 35.3137 35.3137 35.3137 Low frequencies --- 217.2099 316.3451 316.3451
[N(CH3)4]+ |
The frequency file is liked to here
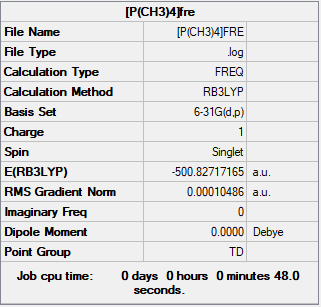
Optimisation and frequency analysis of [P(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000786 0.001800 YES RMS Displacement 0.000303 0.001200 YES Predicted change in Energy=-1.754803D-07 Optimization completed. -- Stationary point found.
Low frequencies --- -0.0028 -0.0025 -0.0011 51.1949 51.1949 51.1950 Low frequencies --- 186.5453 211.3561 211.3561
[P(CH3)4]+ |
The frequency file is liked to here
Charge distribution of [N(CH3)4]+ and [P(CH3)4]+

| Molecular name | [N(CH3)4]+ |
| Charge on central N | -0.295 |
| Charge on C | -0.483 |
| Charge on H | 0.269 |
| Molecular name | [P(CH3)4]+ |
| Charge on central P | 1.666 |
| Charge on C | -1.060 |
| Charge on H | 0.298 |
The different charge distribution on the two compounds could be explained by the different electronegativties of N and P. The small N atom is very electronegative so it carries a negative change of -0.285. Though C is less electronegative than C (electronegativity N:3.04, C:2.55), as each H is bonded to three electropositive H atoms, it carries a much larger negative charge of -0.483.
P atom is less electronegative than C (electronegativity P:2.19, C:2.55), the electron density on P-N bond is dragged towards the C. Therefore, the P carries a positive charge of 1.666. As C dragged electron density from P and H in the same time, it carries a more negative charge of -1.060.
Correct NBO charges calculated and good presentation of the charges and colour range used across both ILs. Good use of relative electronegativities to justify the charges, your answer could be improved by actually comparing the distributions between the two molecules or by considering some other effects. Also watch out for errors when discussing the atoms e.g. comparing C to C. Smf115 (talk) 18:52, 19 May 2019 (BST)
Formal charge on N representing in traditional description in [N(CH3)4]+
In the traditional description of the compound [N(CH3)4]+, the positive 1+ charge is usually positioned at the centre N atom. This positive 1+ charge indicates that the N atom losses one valence electron when forming four bonds: four of the five valence electrons involve in forming the bond and the remaining one is lost.
From the analysis of charge distribution on [N(CH3)4]+, the N carries a negative charge of -0.295 instead of +1. This is because the traditional description ignores the different abilities of different atoms to attract electrons. The electron density is not populated evenly on the molecule. The electronegative N pulls electrodensity from C towards it so it carries negative charges.
In this compound, the positive charge is located on the electropositve H atoms.
An ok discussion about the electronegativities but your explanation of the traditional picture could be clearer and utilise theories such as formal electron counting. Smf115 (talk) 18:53, 19 May 2019 (BST)
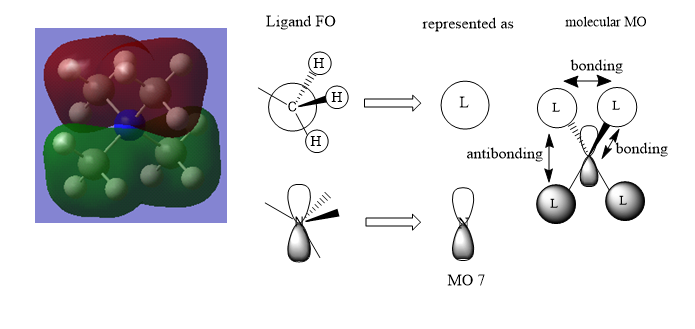
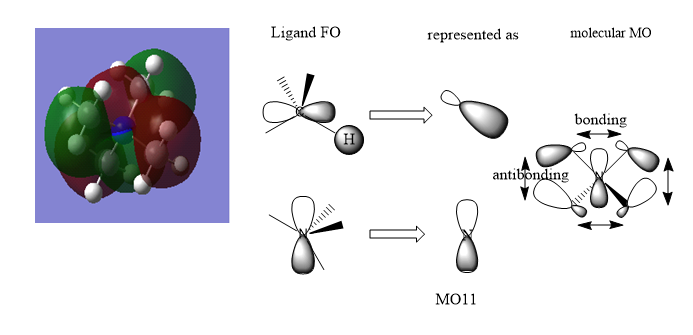
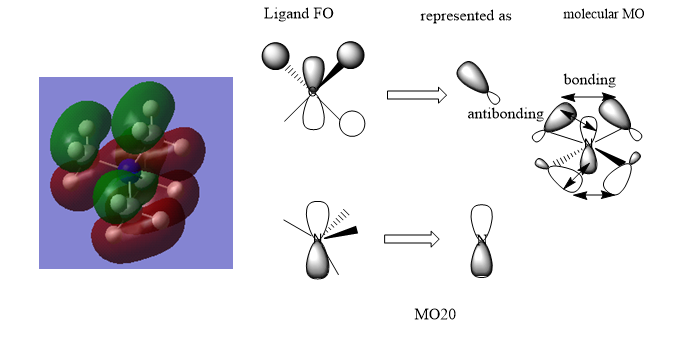
MO Diagrams of [N(CH3)4]+
The MO is overall bonding with very small anti-bonding character via space.
There is some anti-bonding character through space and the bonding character is quite strong due to better overlap.
The anti-bonding character is strong and the bonding character through space is weak in this MO.
Good range of MOs chose and your LCAOs/FOs are correct for MO 7 and 20. However, the ligand FO for MO 11 isn't correct and it might help to consider the BH3 MO diagram in relation to the CH3 which you are representing. Smf115 (talk) 20:48, 21 May 2019 (BST)
Overall, a decent report with a good project section. Smf115 (talk) 20:48, 21 May 2019 (BST)