Rep:Mod:lx921011
The Report
Part 1
The Hydrogenation of Cyclopentadiene Dimer
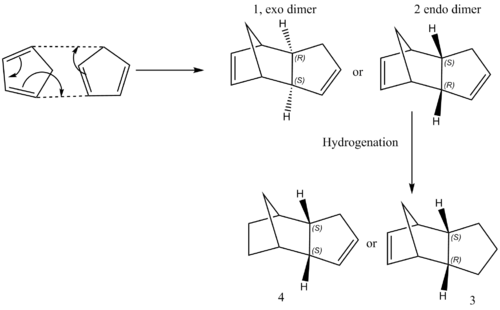
Cyclopentadiene can undergo a cyclodimerisation rapidly at room temperature. Two possible dimers, endo and exo can be formed theoretically. But only endo dimer will be formed, to investigate why the endo dimer is preferred, Molecules 1 and 2 were drawn in ChemDraw and then their geometries were optimised using Avogadro, followed by calculating the minimum energy using the MMF94s force field and conjugate gradients algorithm. The hydrogenation of these two dimers can produce Molecules 3 or 4. In this case, Molecules 3 and 4 were optimised using the same approach described above and their minimum energies were calculated. The results were summarised in the following table.
| Molecules | 1 (kcal/mol) | 2 (kcal/mol) | 3 (kcal/mol) | 4 (kcal/mol) |
|---|---|---|---|---|
| Total Bond Stretching Energy | 3.54206 | 3.46835 | 3.31196 | 2.82316 |
| Total Angle Bending Energy | 30.77174 | 33.18466 | 31.94421 | 24.68527 |
| Total Stretch-Bending Energy | -0.8361 | -2.08239 | -2.10304 | -1.65724 |
| Total Out-of-Plane Bending Energy | 0.01494 | 0.20184 | 0.01300 | 0.00028 |
| Total Torsional Energy | -2.72703 | -2.97281 | -1.47948 | -0.37881 |
| Total VAN DER WAALS Energy | 12.79974 | 12.36226 | 13.63957 | 10.63782 |
| Total Electrostatic Energy | 13.01371 | 14.18788 | 5.11949 | 5.14701 |
| Total Energy | 55.37356 | 58.19085 | 50.44572 | 41.25749 |
The optimised geometries of molecules 1-4 were as follows:
Molecule 1
Pentahelicene |
Molecule 2
Pentahelicene |
Molecule 3
Pentahelicene |
Molecule 4
Pentahelicene |
It can be clearly seen from Table 1 that endo dimer 2 is less stable than the exo dimer 1, indicated by the greater total energy. This is mainly due to the greater total angle bending energy for 2. Because the structure of the Molecule 2 is more bending than the Molecule 1. This is reflected on the structure of 2 that the measured angles show more deviation from the ideal hybridisation angles(120o for sp2 C and 109.5o for sp3 C) than 1. So the reaction is kinetically controlled rather than thermodynamically controlled because the less stable dimer, endo, is formed via a more stable transition state than exo.
It is clear from Table 1 that the Molecule 4 is more stable than the Molecule 3, mainly contributed from the bending energy, indicating that the Molecule 3 has more bending character. This can also be interpreted by the measured angles on the carbon frameworks using the same approach described above. So the Molecule 4 will be the main product of the hydrogenation if this reaction is under thermodynamic control.
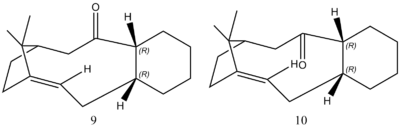
Molecules 9 and 10, differing in only the position of the carbonyl group, can be converted on to each other standing, meaning they are antropisomers.
To decide which antropisomer is more stable, their geometries were optimised using the same force field and the algorithm as above. To rationalise why Molecules 9 and 10 react slowly upon hydrogenation, it is necessary to calculate the optimised energies of their parent hydrocarbons and compare the energies with molecules 9 and 10 to figure out if the alkenes are more stable than the parent hydrocarbons as suggested by Maier et al[1]. The optimised structures of 9, 10 and their parent hydrocarbons were shown below.
Molecule 9
Pentahelicene |
Molecule 10
Pentahelicene |
9 Parent Hydrocarbon
Pentahelicene |
10 Parent Hydrocarbon
Pentahelicene |
The results for the energy calculations were summarised in the following table:
| Molecules | 9 (kcal/mol) | 10 (kcal/mol) | 9 Parent Hydrocarbon (kcal/mol) | 10 Parent Hydrocarbon (kcal/mol) |
|---|---|---|---|---|
| Total Bond Stretching Energy | 7.68361 | 7.79091 | 6.90068 | 6.48297 |
| Total Angle Bending Energy | 28.29282 | 19.06655 | 32.19675 | 23.99297 |
| Total Stretch-Bending Energy | -0.07086 | -0.14145 | 0.31388 | 0.40526 |
| Total Out-of-Plane Bending Energy | 0.97127 | 0.95445 | 0.24153 | 0.10704 |
| Total Torsional Energy | 0.19344 | 3.80810 | 9.47131 | 12.30089 |
| Total VAN DER WAALS Energy | 33.16640 | 34.95734 | 32.64334 | 32.77112 |
| Total Electrostatic Energy | 0.30111 | -0.06079 | 0.00000 | 0.00000 |
| Total Energy | 70,53780 | 66.37512 | 81.76750 | 76.06026 |
It can be seen from Table 2 that Molecule 10 has more stability than Molecule 9, which is mainly contributed by the bending energy. Because the measured angles show less deviation to ideal hybridised angles, probably caused by the different conformations of the six-membered rings, resulting in the less bending energy for 10. Also, Table 2 reveals that the total energies of both hydrocarbons are higher than the alkenes, denoting the alkenes are more thermodynamically stable than the hydrocarbons, causing the slow hydrogenation to be observed. This is possibly due to an unknown stabilisation of the tri-substituted bridgeheaded alkenes which have high strain energies according to Maier et al[1].
References
- ↑ 1.0 1.1 W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
Spectroscopic Simulation using Quantum Mechanics
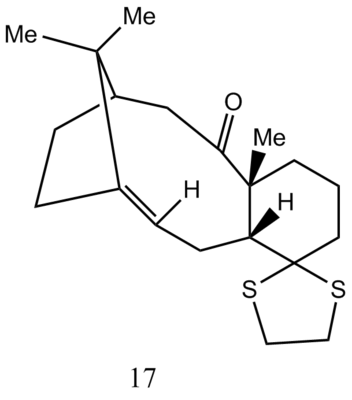
Avogadro was used to optimise Nolecule 17 and calculate the minimum energy. Then, Gaussian was employed to calculate the geometry at the density functional level under B3LYP theory and 6-31G(d,p) basis, with chloroform as a solvent to compute13C and 1H NMR spectra of 17. To visualise the NMR spctra, TMS B3LYP/6-31G(d,p) Chloroform was chosen to be the reference value. It should be mentioned that the chemical shifts of the carbons attached to heavy elements such as sulfur were estimated to be corrected for -3 ppm[1][2] due to Spin-orbital coupling errors. For the carbonyl carbons, δcorr = 0.96δcalc + 12.2 was used to correct chemical shifts[1][2]. Also, the chemical shifts for hydrogens on a methyl group(37,38,39; 40,41,42; 43,44,45) is the average shift of the three protons due to fluxionality. The results (DOI:10042/25704 ) for 1H NMR and 13C NMR were summarised in Tables 3 and 4.
Molecule 17 after optimised by Avogadro and Gaussian
Pentahelicene |
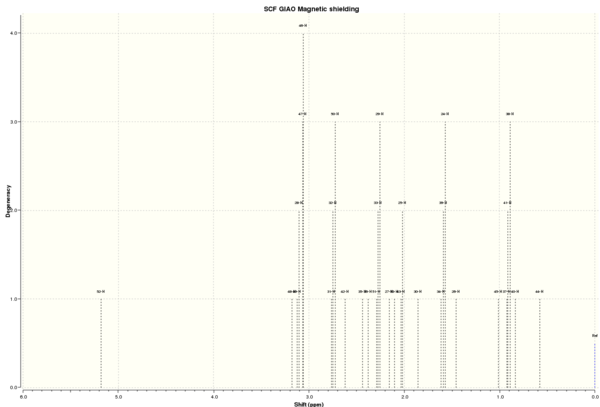
| Shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 5.1826135842 | 1.0000 | 52 |
| 3.1775718770 | 1.0000 | 48 |
| 3.0867242905 | 4.0000 | 49,28,47,46 |
| 2.7442862001 | 3.0000 | 31,32,50 |
| 2.4371215980 | 1.0000 | 35 |
| 2.3781086250 | 1.0000 | 36 |
| 2.2733023513 | 3.0000 | 51,33,29 |
| 2.1561097653 | 1.0000 | 27 |
| 2.1033034684 | 1.0000 | 53 |
| 2.0250487133 | 1.0000 | 25 |
| 1.8531435177 | 1.0000 | 30 |
| 1.5897769472 | 2.0000 | 34,24 |
| 1.4555208583 | 1.0000 | 26 |
| 1.454346942 | 3.0000 | 40,41,42 |
| 1.205463467 | 3.0000 | 43,44,45 |
| 1.13563703 | 3.0000 | 37,38,39 |
| Shift (ppm) | Corrected Shift(ppm) | Degeneracy | Atoms |
|---|---|---|---|
| 216.2731821442 | 219.8222549 | 1.0000 | 14 |
| 144.8649259001 | N/A | 1.0000 | 9 |
| 124.7173392829 | N/A | 1.0000 | 8 |
| 91.3532315558 | 85.3532316 | 1.0000 | 5 |
| 60.9629515076 | N/A | 1.0000 | 4 |
| 57.0836015519 | N/A | 1.0000 | 3 |
| 52.4621178288 | N/A | 1.0000 | 12 |
| 51.4861493783 | N/A | 1.0000 | 16 |
| 46.9217338887 | N/A | 1.0000 | 13 |
| 43.8717176800 | 40.8717176800 | 1.0000 | 23 |
| 41.9473921387 | 38.9473921387 | 1.0000 | 22 |
| 41.8769214269 | N/A | 1.0000 | 6 |
| 35.4352634681 | N/A | 1.0000 | 7 |
| 31.1973052267 | N/A | 1.0000 | 11 |
| 28.9403195420 | N/A | 1.0000 | 2 |
| 28.3570358061 | N/A | 1.0000 | 19 |
| 27.3749849860 | N/A | 1.0000 | 10 |
| 26.3304687301 | N/A | 1.0000 | 17 |
| 24.5524117004 | N/A | 1.0000 | 1 |
| 19.7768691476 | N/A | 1.0000 | 18 |
By comparing computed chemical shifts of the computed 1H NMR spectrum with the the literature values[3], it can be deduced that 1H NMR generally shows larger chemical shifts compared to the literature values, with the proton 52 and those protons in the methyl groups being more deshielded. This means the calculated 1H NMR spectrum does not match well with the exoerimental spectrum. To compare 13C spectra, a bar chart of the difference in ppm between the computed spectrum and the experimental spectrum against the nucleus number was plot using Excel.
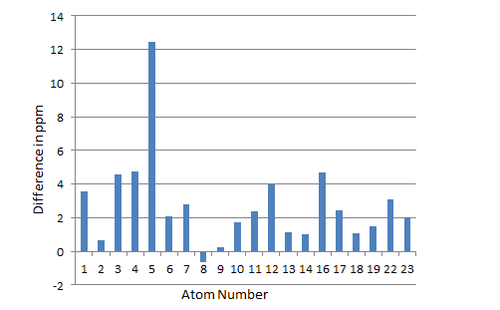
It is clear from Figure 6 that there is a generally great match between the computed spectrum and the experimental spectrum. Again, the calculated chemical shifts tend to be greater than experimental chemical shifts. The difference in chemical shifts can be caused by the different solvents used(C6D6 for the experimental and CHCl3 for the computed spectrum) , different temperatures and computational calculations etc. Almost every difference is within 5 ppm except the difference for Nucleus 5. Considering the excellent performance of the calculation, this is probably due to wrong assignments of the literature values. This kind of analysis is impossible for 1H NMR as the literature values reveals that there is a series of multiplet between 2.80 ppm -1.35 ppm, which is not specified enough.
The thermodynamic properties of the Molecule 17 were summarised as follows:
| Terms | Energy (Hartree/Particle) |
| Zero-point correction | 0.468396 |
| Thermal correction to Energy | 0.489751 |
| Thermal correction to Enthalpy | 0.490695 |
| Thermal correction to Gibbs Free Energy | 0.422008 |
| Sum of electronic and zero-point Energies | -1651.414385 |
| Sum of electronic and thermal Energies | -1651.39303 |
| Sum of electronic and thermal Enthalpies | -1651.392086 |
| Sum of electronic and thermal Free Energies | -1651.460773 |
Reference
- ↑ 1.0 1.1 R. Jain, T. Bally, P.R. Rablen,J. Org. Chem., 2009, 74, 4017–4023 DOI:10.1021/jo900482q
- ↑ 2.0 2.1 DOI:10.1021/jo900408d ,Applet,DOI:10.1021/ja105035r ,Blog commentary
- ↑ L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. RogersJ. Am. Chem. Soc. , 1990, 112, 277-283. DOI:10.1021/ja00157a043
Part 2
The Crystal Structures of the Shi Catalyst and the Jacobsen Catalyst
Shi and Jacobsen catalysts, as shown in Figures 7 and 8, were used in asymmetric epoxidation of alkenes. Their crystal structures, which were summarised in Figures 9 and 10, were found using the Conquest and the Mercury programs from the Cambridge Crystal Database.
The crystal structures of Shi[1] and Jacobsen [2] catalysts were shown as follows.
Shi Catalyst
Pentahelicene |
Jacobsen Catalyst
Pentahelicene |
For each anomeric centre on the shi catalyst, one C-O bond is shorter than the sum of the covalent radii(142 pm) while another is longer, caused by the donation of the lone pair from the oxygen to the C-O σ * orbital, shortening one C-O bond and lengthening another C-O bond due to more electron density on the anti-bonding orbital. Which bond is shortened is determined by the inductive withdrawing effect of the carbonyl group. This was illustrated in Figures 9 and 10.
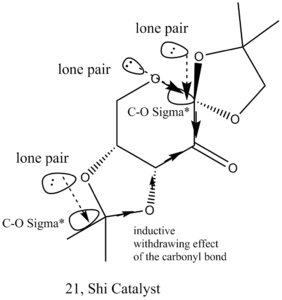
For the Jacobsen catalyst,it is interesting to see that this molecule adopts a square based pyramidal structure at the metal centre, with the chloride at the axial position. As each ligand except the chloride is connected on a ring, so it is better for all of the donor atoms to be on the equatorial positions to minimise the torsional strain rather than adopting a trigonal pyramidal structure, where it is impossible to have the four donor atoms co-planar. Thus, the square based pyramidal conformation, which is lower in energy, is preferred. In addition, a number of H...H, C...C and C...H short contacts were found between two adjacent t-butyl groups on the ring. This lowers the total energy of the square based pyramidal conformation.
References
- ↑ Zhi-Xian Wang, S.M.Miller, O.P.Anderson, Yian Shi, J.Org.Chem. , 2001, 66, 521. DOI:10.1021/jo001343i
- ↑ J.W.Yoon, T.-S.Yoon, S.W.Lee, W.Shin, Acta Crystallogr.,Sect.C:Cryst.Struct.Commun. , 1999, 55, 1766. DOI:10.1107/S0108270199009397
The Calculated NMR Properties of the Epoxides
Again, to calculate the NMR spectra,their geometries were optimised using the same approach as described for Molecule 17. Below are the results:
Epoxide 2 (RR) after optimisation
Pentahelicene |
Epoxide 4 (RR) after optimisation
Pentahelicene |
Epoxide 2 (SS) after optimisation
Pentahelicene |
Epoxide 4 (SS) after optimisation
Pentahelicene |
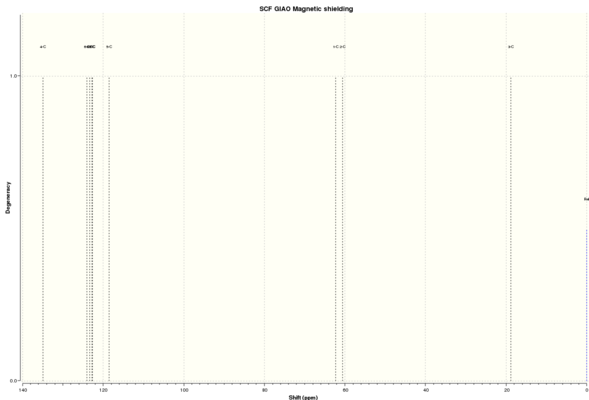
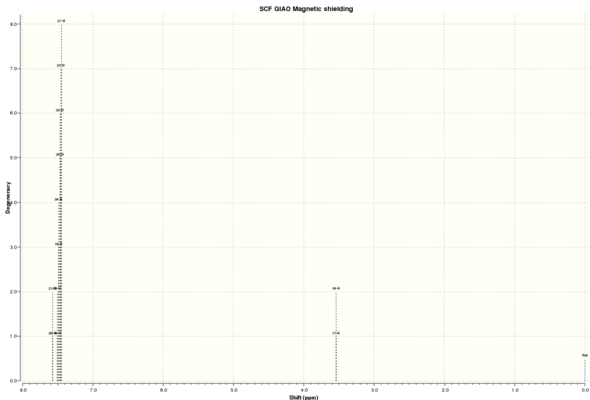
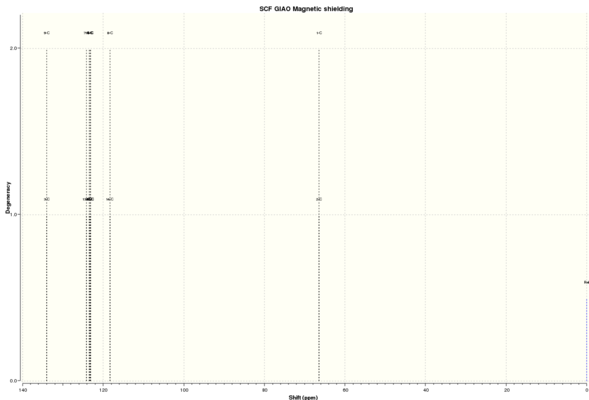
| C NMR 3 | ' | ' | C NMR 4 | ' | ' | H NMR 3 | ' | ' | H NMR 4 | ' | ' |
| Shift (ppm) | Degenracy | Atom | Shift (ppm) | Degenracy | Atom | Shift (ppm) | Degenracy | Atoms | Shift (ppm) | Degenracy | Atom |
| 134.9756393 | 1 | 4 | 134.0861502 | 2 | 3,9 | 7.491171152 | 3 | 20,17,19 | 7.570471208 | 2 | 26,21 |
| 124.0725625 | 1 | 6 | 124.2196281 | 2 | 13,7 | 7.421508417 | 1 | 18 | 7.479071045 | 8 | 18,23,19,24,25,20,22,27 |
| 123.3280498 | 1 | 8 | 123.5176328 | 2 | 5,11 | 7.307305368 | 1 | 16 | 3.537301032 | 2 | 17,16 |
| 122.7955089 | 1 | 9 | 123.2128167 | 2 | 12,6 | 3.414768141 | 1 | 12 | |||
| 122.7269517 | 1 | 1 | 123.0779464 | 2 | 10,4 | 2.787810289 | 1 | 11 | |||
| 118.4863933 | 1 | 5 | 118.2640383 | 2 | 14,8 | 1.6782286 | 1 | 14 | |||
| 62.31988795 | 1 | 7 | 66.4245522 | 2 | 2,1 | 1.58729176 | 1 | 15 | |||
| 60.57670932 | 1 | 2 | 0.716946021 | 1 | 13 | ||||||
| 18.83765744 | 1 | 3 | |||||||||
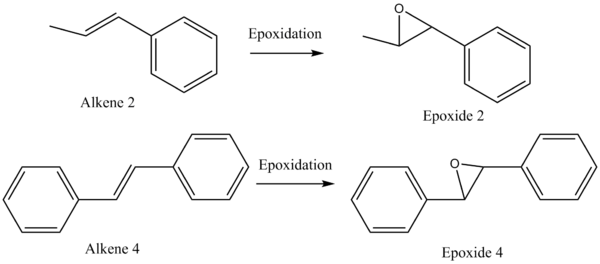
The Assignment of the Absolute Configurations for Alkenes 2 and 4
The epoxidation of alkenes is stereospecific with respect to alkenes. This means the E/Z configuration remains the same during the epoxidation. However, as the epoxidation proceeds via a syn addition mechanism, the stereochemistry of the final product can be classified into RR and SS for the reactions shown in Figure 11. Therefore, analytical techniques are needed to detect the stereochemistry of the final product. So the optical rotatory powers for RR and SS epoxides can be searched and computed to provide information on the absolute configurations of the products.
First of all, Reaxys was used to search the literature values for the optical rotatory powers for SS and RR epoixdes. Then, computational analyses were conducted by Gaussian using CAM-B3LYP Method, 6-311++g(2df,p) Basis and chloroform as a solvent to calculate the optical rotatory powers at 589 nm and 365 nm based on the optimised structures of Epoxides 3 and 4. The results were presented in Tables 7 and 8.
| Epoxides | 2,SS[1] | 2,RR[2] | 4,SS[3] | 4,RR[2] |
|---|---|---|---|---|
| Concentration (g/100ml) | 1 | 0.32 | 0.56 | 0.73 |
| Enantiometric Excess (%) | 99 | 90 | 89 | 97 |
| Solvent | CHCl3 | CHCl3 | CHCl3 | CHCl3 |
| Optical Rotation | -41.8o | 44.3o | -205.2o | 334.6o |
| Wavelength (nm) | 589 | 589 | 589 | 589 |
| Temperature | 25oC | 25oC | 20oC | 25oC |
| 2, SS DOI:10042/25737 | 2, RR DOI:10042/25042 | 4, SS DOI:10042/25738 | 4, RR | |
|---|---|---|---|---|
| αd at 589 nm | -48.50o | 46.77o | -322.38o | 298.28o |
By comparing the values of the optical rotation for SS and RR epoxides at 589 nm, it is clear that the values calculated using CAM-B3LYP method match both of the signs and the magnitudes of experimental values, although showing some extend of deviation. Thus, it can be concluded that the literature assignments were right and the calculation method is reliable.
Is is also necessary to compare the energies in transition states for Shi epoixdation of β-methyl styrene to investigate which epoxide will be the major product by analysing the computed thermodynamic properties to calculate the value of K between RR and SS epoxides and hence the enantiomeric excess by using ΔG= -RTlnK. The results of the analysis were presented in the following table.
| Free Energies (Hartrees) | Free Energies (Hartrees) | |
| Transition State | RR | SS |
| 1 | -1343.02297 | -1343.017942 |
| 2 | -1343.019233 | -1343.015603 |
| 3 | -1343.029272 | -1343.023766 |
| 4 | -1343.032443 | -1343.024742 |
| Average ΔG | -1343.02598 | -1343.020513 |
| Free Energy Difference (RR-SS) | -0.00546625 | |
| K | 326.9 | |
| Relative Population (%) | 99.7 | 0.3 |
| Enantiomeric Excess | 99.4 |
Is is clear from Table 9 that all RR transition states are more stable than SS transition states. Also, the enantiomeric excess of the RR product relative to the SS product reveals a great match to the literature values in Table 7, confirming the literature assignment of the absolute configuration for Shi oxidation of the β-methyl styrene. To conclude, RR epoxide will be the major product for this reaction and the calculation method is reliable on predicting the results for the epoxidation using Shi catalyst.
In addition, assigning the absolute configuration by the vibrational circular dichroism (VCD) and the electronic circular dichroism (ECD) is feasible. However, as there is no choromophore in the Epoxides 2 and 4, so the ECD spectrum, which is the UV/Vis spectrum, is not suitable for the assignment. VCD spectrra of RR and SS epoxides could be chosen to help the assignment. The VCD spectra were presented in the following graphs.
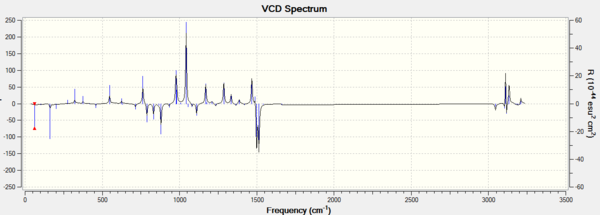
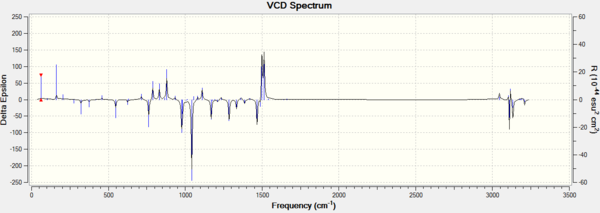
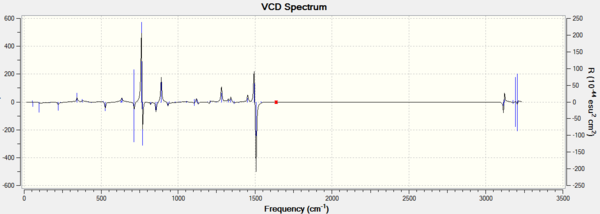
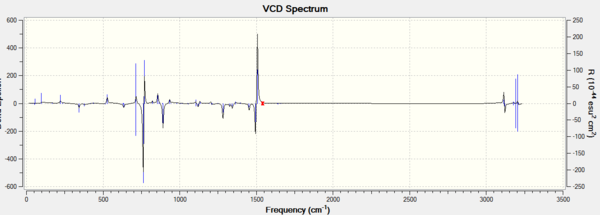
It is interesting to see that VCD spectra of a pair of enantiomers are also mirror images of each other, due to two complete and opposite vibrational environments. So it is helpful to the assignment of the absolute configurations for two epoixdes under investigation. However, measuring VCD spectra is impossible in the department.
References
NCI Analysis for the Transition State
The first transition state of R,R series for Shi epoxidation of β-methyl styrene was chosen to undergo NCI analysis.
Orbital |
It can be seen from the green region, which is attractive, that the active catalyst binds to the substrate via the oxygen atoms labelled in yellow. So the substrate must orient itself in a manner shown in the NCI analysis to mixmise this interaction. In this case, the two adjacent oxygen atoms in yellow must direct to the methyl group of the alkene and the other oxygen atom in yellow must direct to the phenyl group of the alkene. This is the origin of the stereoselectivity.
QTAIM Analysis for the First Transition State of RR Series of Shi Epoxidation
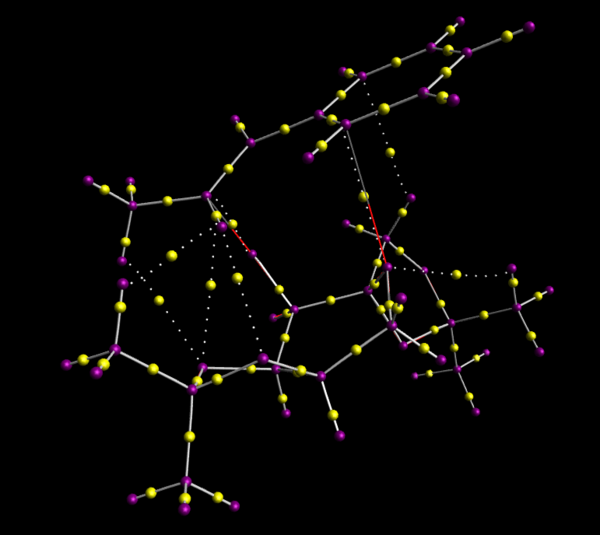
The orientation of Shi catalyst relative to the alkene was confirmed by QTAIM analysis as shown in Figure 20
New Candidates
Through Reaxys, two potential epoxides and the corresponding alkene can be found.
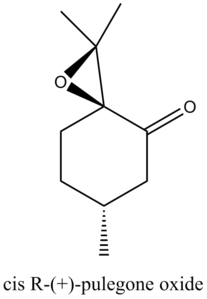
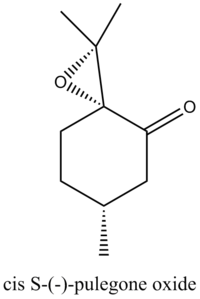
The optical properties of the epoxides were summarised in the following table.
| R | S | ||
| Concentration (g/100ml) | 0.03 | 0.03 | |
| αd | 853.9o | -1177.9o | |
| Wavelength (nm) | 324 | 327 | |
| Solvent | EtOH | EtOH | |
| Temperature | 25oC | 25oC |
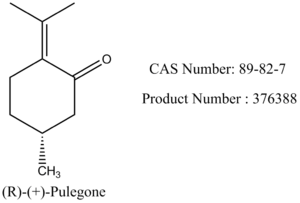
The alkene can be found in the in the catalogue of Sigma-Aldrich, with Product Number and CAS Number included included in Figure 23. So it is a feasible alkene to be investigated.
Reference
- ↑ Reusch; Johnson Journal of Organic Chemistry 1963, 28, 2557
Pros and Cons of Softwares
Recongnising pros and cons of softwares used in comutational chemistry is very important for us to be able to choose the appropriate software for analysis, hence saving time and increasing the efficiency in computational labs.
Cons
Avogadro: Sometimes unstable; The Result for QTAIM cannot be saved when the analysis was finished; QTAIM analysis crashes in Windows 7; Losing stereochemistry when open the molecules drawn in ChemDraw.
ChemBio3D: Moving an atom manually leaves grey shadow on the screen;Longer time for an optimisation than Avogadro.
Gaussview: Opening the program is very slow in Windows; Some calculation results such as the total energy of the system and the optical rotation cannot be viewed using this program; Sometimes crashes.
Pros
Avogagro: Shorter time for an optimisation process; Can be extended to external softwares easily.
ChemBio3D: ChemDraw can be used to draw molecules in ChemBio3D, so the stereochemistry can be adjusted immediately.
Gaussview: Can undergo various of calculations such as MO, UV Spectrum and NMR spectrum while other softwares cannot.
