Rep:Mod:lp1990
Module 1: Modelling with Molecular Mechanics
Introduction
The molecular mechanics (MM) approach tries to study molecular properties such as strain, hindrance and stability of molecular conformation, without the need of solving wave equations; it studies them in terms of bond properties thus not using quantum mechanical approaches. MM assumes that the energy of a molecular system comes from 5 additive and non-interacting terms:
- The sum of all diatomic bond stretches (each expressed as a simple Hookes law potential). Stretch
- The sum of all triatomic bond angle deformations (also a simple Hookes law potential). Bend
- The sum of all tetra-atomic bond torsions (a cosine dependance).Torsion
- The sum of all non-bonded Van der Waals repulsions (using a simple 6/12 potential). Van Der Waals
- The sum of all electrostatic attractions of individual bond dipoles. Dipole-Dipole
This terms were found and compared, using ChemBio 3D in order to predict the geometry and regioslectivity of:
- the hydrogenation of a cyclopentadiene dimer
- the nucleophilic addition to two different pyridinium rings
- the conformation/atropisomerism of a large ring ketone intermediate in one synthesis of the anti-cancer drug Taxol
The Hydrogenation of Cyclopentadiene Dimer
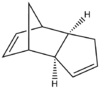
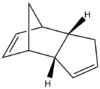
Cyclopentadiene is often used with ethylene in the synthesis of norbornene, which goes via a Diels-Alder 4πs + 2πs cycloaddition reaction. However, this process often involves a side reaction which is the dimerization of cyclopentadiene. This is the result of the 4πs + 2πs cycloaddition of two cyclopentadienes and produces two diasteroisomers: the exo isomer and the endo isomer.
According to theory, the Endo isomer is produced in preference during a reaction. This was reported experimentally by Baldwin in 1966. Baldwin also found that the Exo isomer could be obtained if the Endo product was heated to high temperatures. However, nowadays this can also be investigated using MM. The energies, split into their components, for the two isomers are reported in the the table below.
Table 1: Comparison of the energies of the exo and endo dimers
| Energies/ kcal mol-1 | |||||||
| Stretch | Bend | Stretch-Bend | Torsion | Van der Waals | Dipole-Dipole | Total | |
| Exo isomer | |||||||
| Endo isomer | |||||||
From table 1 it can be seen that the Endo isomer is higher up in energy than the Exo isomer, thus showing that the Exo isomer is thermodynamically more favored. The energy difference between the two is BOOOOO kJmol, mainly due to a higher torsion strain. The 1,4 strain in the endo product can easily be seen in the figure below.
The fact that the Exo product is thermodynamically more favourable might at first seem to contradict the theory put forward experimentally by Baldwin, however, the discrepancy between the two can be explained in terms of thermodynamic vs. kinetic control. These two conditions yield two different products. Kinetic control, is non reversible and will form the product that's produced in the shortest time (faster rate) i.e. the one with the lowest activation energy. Thermodynamic control is reversible and will produce the product with the lowest energy.
The Endo product is therefore the kinetic product and was found by Baldwin to be the major product formed because he carried out the experiment under kinetic conditions. This must mean that the Endo product has the lowest activation barrier. This statement can be rationalized on the bases of the fact that the Endo transition state (TS) can enjoy further stabilization due to a favourable orbital overlap between the HOMO orbitals of one ring and the LUMO orbitals at the back of the other ring.This doesn't exist in the Exo transition state, thus leading the Exo TS to be higher up in energy.
Hydrogenation of the endo isomer of cyclopentadiene dimer.
The hydrogenation of the endo isomer occurs in two different steps. Initially, only one double bond is attacked by hydrogen and only after prolonged hydrogenation the fully saturated product is given. We can use MM to predict which double bond will be attacked first, by comparing the energies of structure 1 and structure 2.
 |
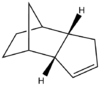 | |||
Table 2: Comparison of the energies of the hydrogenation products
| Energies/ kcal mol-1 | |||||||
| Stretch | Bend | Stretch-Bend | Torsion | Van der Waals | Dipole-Dipole | Total | |
| Structure 3 | |||||||
| Structure 4 | |||||||
