Rep:Mod:ll4012
Introduction
This computational lab course primarily involves an investigation into pericyclic transition states. The course will be completed using Gaussview 5, a software which usies approximations to solve the Schrödinger equation for various molecules. The course will use various methods to achieve similar results. First, these methos will be used to optimise transition states and also model the reaction for the Copt rearrangement. The program will then be used to model more complicated diels alder reactions. By the end the benefits of using a program such as gaussview to gain an insight into the molecular interactions involved in preicyclic transition states will have bee seen.
Cope Rearrangement Tutorial
Introduction
The Cope rearrangement is a [3,3]-sigmatropic rearrangement involving a 1,5-diene[1]. The pericyclic reaction proceeds via either a chair or a boat transition state and is under thermal control.

The chair and boat transition states resemble that of the two conformers of cyclohexane, in this, the chair conformation is the lowest energy, due to it being less sterically hindered.

Hexadiene
Hexadiene was constructed on gaussview in an "anti" conformation for the central 4 carbon atoms. The structure was optimised using the Hartree-Fock method and 3-21G basis set with 250MB of memory.
As shown in the above table, the energy of the molecule was calculated to be -231.69260235 au. The symmetrize function in gaussview was used to determine the point group as C2. This coincides with the data supplied in Appendix 1 in the brief, which identifies the conformer as anti1. The .log file can be accessed here File:1-5-HEXADIENE ANTI3 LAL.LOG
A gauche conformation of 1,5-Hexadiene was then optimised using the same method and basis set.
The energy of this conformation is slightly higher than the anti linkage, at -231.6915303 au. The point group of the conformer was shown to be C2. This data complies with that in Appendix 1, which identifies the conformer as gauche4. The .log file can be accessed at File:1-5-HEXADIENE GAUCHE3 LAL.LOG.
The energy of this conformation implies that the anti conformer is a lower energy. This is most probably because it is the least sterically crowded of the two. as the other anti-conformers are more sterically crowded than the anti conformer shown, either this is the most stable format, or another gauche conformation is. If it is a gauche conformer, it will most likely not be an eclipsed configuration, and the allyl groups will most probably be pointing away from each other. Using this information the following molecule was constructed and optimised.
This gave the lowest energy so far, at -231.69267 au. The point group was given as C1. This data, and the image, corresponds to the data in Appendix 1, labelled as gauche3. According to theory, this is what the activation energy should be measured from. The .log file can be accessed at File:1-5-HEXADIENE GAUCHE MIN LAL.LOG
The anti2 conformation of 1,5-hexadiene was drawn and optimised, again using the HF/3-21G method and basis set.
The energy was given as -231.69253528 au. The point group was also given as Ci. This coincides with the information given in the appendix table. The .log file can be accessed at File:1-5-HEXADIENE ANTI CI LAL.LOG.
Therefore an optimisation was run using a better method and basis set (DFT/6-31G*)
The energy given is largely different to that shown by the 3-21G basis set, with the difference being 2.93064 au. This is a massive amount of energy (1839.0 kcal/mol), and proves that energies cannot be compared when different basis sets have been used. The .log file can be accessed at File:1-5-HEXADIENE ANTI CI 6-31G LAL.LOG.
Bond length analysis was also carried out to highlight the differences between the two basis sets. If we take 6-31G* as the more accurate basis set, we can see that 3-21G underestimates the terminal C=C, as well as the C-H bonds. It however overestimates the bond lengths of the more central C-C bonds.
| 1, 5 Hexadiene Anti Ci Bond Lengths (Å) | |||
|---|---|---|---|
| Basis Set | 3-21G | 6-31G* | Difference |
| H2C=CH- | 1.3161 | 1.3370 | 0.0209 |
| H-CH=CH- | 1.0734 | 1.0891 | 0.0157 |
| H2C=C-H- | 1.0769 | 1.0923 | 0.0154 |
| =HC-CH2- | 1.5089 | 1.5048 | -0.0041 |
| -H2C-CH2- | 1.5528 | 1.5485 | -0.0043 |
| -HC-H- | 1.0848 | 1.0984 | 0.0136 |
| -HC-H- | 1.0857 | 1.1001 | 0.0145 |
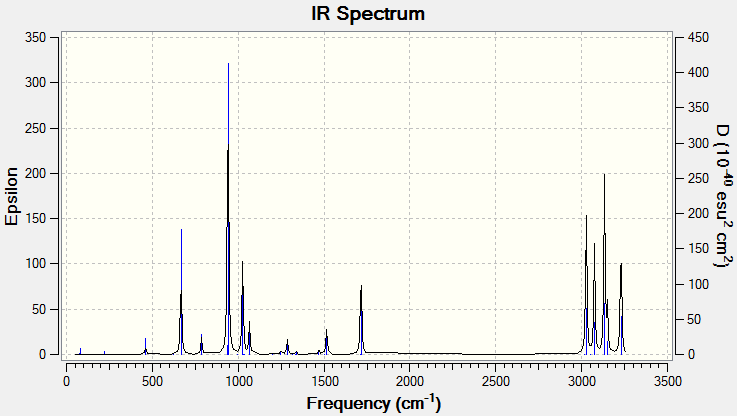
A frequency calculation was then carried out on the optimised structure, with the same basis set and method.
The vibrational analysis showed no negative vibrations, and produced an IR spectrum. Notable peaks include =C-H2 bends (940.7 cm-1, C=C asymmetric stretch (1715.4 cm-1) and various C-H symmetric and asymetric stretches (3026.9-3229.3 cm-1).
The .log file can be accessed at File:HEXADIENE FREQ DFT1 LAL.LOG.
Sum of electronic and zero-point Energies= -234.481085 Sum of electronic and thermal Energies= -234.473729 Sum of electronic and thermal Enthalpies= -234.472785 Sum of electronic and thermal Free Energies= -234.512661
Chair and Boat Transition Structures
Chair
A delocalised CH2CHCH2 fragment was drawn and optimised using gaussview. The hartree-fock method was used in conjunction with the 3-21G basis set. The fragment was copied twice into a new window. The two fragments were copied on top of each other, so that the terminal carbons were approximately 2.2 Å apart and they were inverted against each other. This was saved as a "guess" input file.
An Opt+Freq calculation was run using the HF/3-21G method/basis set and the opt=noeigen. the optimisation was also optimised to a TS(berny) as opposed to a minimum.
| Chair TS Optimisation (Part 1) | |
|---|---|
| |
| File Type | .log |
| Calculation Type | FOPT |
| Basis Set | 3-21G |
| Energy (au) | -231.6193224 |
The calculation returned an optimised TS (above), with an imaginary vibrational frequency of -817.8 cm-1. This vibration was an asymmetric stretch, indicating that one bond is formed at a time, as opposed to both simultaneously. This is representative of the cope rearrangement. The energy was reported to be -231.6193224 au. The file can be accessed at File:CHAIR TS OPT FREQ1 LAL.LOG.
This guess input file was then modified to freeze the potential terminal carbon atom bonds at 2.2 Å. An optimisation was then run to find a minimum. The result was similar to the picture above, but with the the terminal carbon distances at 2.2 Å. File:CHAIR TS FREEZE OPT II LAL.LOG
The freeze instruction was then replaced with the derivative instruction and the structure was optimised to a TS(berny) using the same basis set as before.
The result is similar to the previous structure, but with the previously frozen bond lengths now at a length of approximately 2.02 Å. The energy of the transition state was reported to be -231.6193218. File:CHAIR TS DERIV2 LAL.LOG
| Chair TS Optimisation (Part 2) | |
|---|---|

| |
| File Type | .log |
| Calculation Type | FOPT |
| Basis Set | 3-21G |
| Energy (au) | -231.6193218 |
Boat
An optimised structure of Ci 1,5-hexadiene was used as the product and the reactant. The atoms were labelled to demonstrate the fact that there had been a rearrangement between the two structures.
A QST2 opt+freq calculation was then run. The oputput looked similar to that of the chair transition state, but with different bonds associated (below). The .log file can be found at File:1-5-HEXADIENE ANTI DUAL4 LAL.LOG
| Boat TS Optimisation Failed | |
|---|---|
 |
|
To solve this the conformation was changed by altering the dihedral angle across the four central carbon atoms to 0°. The central three carbon atoms (C2-C3-C4 & C3-C4-C5) bond angle was also reduced to 100°. This changed the conformation from having an anti- linkage to an eclipsed conformation across the central 2 C atoms. This is much closer to the boat structure. The QST2 opt+freq was run again.
| Boat TS Optimisation Successful | |
|---|---|
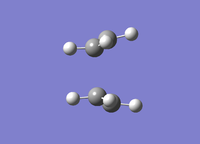 |
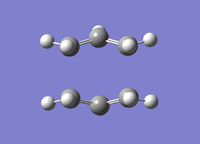
|
The transition state was optimised successfully. The vibrational analysis gave rise to an imaginary frequency at -839.9 cm-1. Like the chair transition state, the bonds are formed asymmetrically. This is representative of the cope rearrangement. The .log file can be accessed at File:BOAT TS OPT FREQ2 LAL.LOG.
The same transition state was then optimised using the QST3 method. A guess boat structure was used in conjunction with the original anti conformers for products and reactants. The two transition states are compared below.
| Boat TS Method Comparison | ||
|---|---|---|
| QST2 | QST3 | |
| Frequency (cm-1) | -893.9 | -894.0 |
| C-C Bond Length (Å) | 1.3815 | 1.3815 |
| Terminal Bond Length (Å) | 2.1400 | 2.1403 |
| C-C-C Bond Angle (°) | 121.67 | 121.68 |
| Energy (au) | -231.6028020 | -231.6028024 |
As can be expected, the two transition states are very similar in characteristics. The differences are most probably due to inaccuracies in the program. The .log file for the QST3 calculation can be found at File:BOAT QST3 OPTFREQ4 LAL.LOG.
IRC Calculations
In order to find out which conformation causes the chair transition structure an intrinsic reaction coordinate calculation was run. The calculation was run in the forward direction and the number of points along the IRC was set to 50.
| Chair TS IRC Conformers | |
|---|---|
| Minimum (43) | Final Conformer (44) |
 |
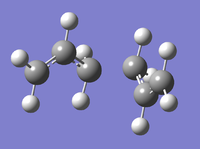
|
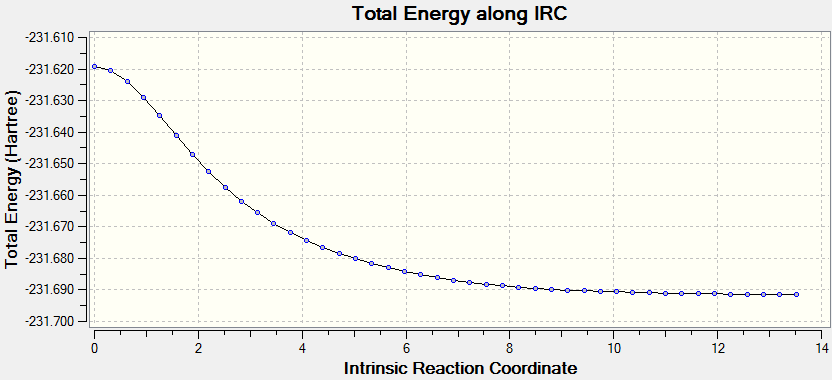
The result returned 44 intermediate geometries, of which the 43rd is situated at a minimum. (The 44th is also situated on the minimum but the root mean square gradient along the path of the IRC is at a minimum on the 43rd conformation. This is also shown in the image, as gaussview defines bonds by length, the 43rd conformation shows the correct bond lengths for a stable minimum. the final conformation however, does not, instead resembling the transition state more. This is shown by the IRC plot below. The energy of this conformation was reported as -231.691530 au. The .log file can be accessed at File:IRC CHAIR FRI I LAL.LOG.
The calculation was run again, using conformer 43, with 100 points specified, as opposed to the 50 run before. The result heralded 44 results again. This implies that the minimum has been found. The final conformer in this result has the energy -231.691579 au. This is marginally lower than the minimum from the previous calculation. The structure is identical.
With regards to assigning the structure, it looks like gauche2 in Appendix 1, and the symmerty matches as well (C2), however the energy is different. It is only slightly different however (0.00009 au), meaning it could well be this structure. The previous conformer matched the energy of the gauche4 in energy and symmetry exactly, however it did not look the same. Judging by the appearance and the very marginal difference in energy, I would conclude that the conformation arrived at by the IRC analysis is the gauche2 conformer.
Activation energies
The activation energy is the difference in energy between the lowest conformer of reactant and the transition state. In order to calculate the activation energy of a reaction, the basis sets used must be the same for all structures used. Therefore the chair and boat conformations were reoptimised (opt+freq) using the B3LYP method and the 6-31G* basis set. This new basis set gave rise to new imaginary frequencies. The boat transition structure gave a new imaginary vibration at -530.9 cm-1 and the chair transition state returned a frequency of -565.7 cm-1.
| Chair and Boat Optimisations | ||
|---|---|---|
 |

| |
| Boat | Chair | |
| File Type | .log | .log |
| Calculation Type | FREQ | FREQ |
| Basis Set | 6-31G(d) | 6-31G(d) |
| Energy (au) | -234.543093 | -234.556983 |
| File | File:BOAT 6-31GD OPTFREQ LAL.LOG | File:CHAIR 6-31G OPTFREQ LAL.LOG |
The thermochemical data for the boat transition structure (6-31G*) is displayed below.
Sum of electronic and zero-point Energies= -234.402336 Sum of electronic and thermal Energies= -234.396002 Sum of electronic and thermal Enthalpies= -234.395058 Sum of electronic and thermal Free Energies= -234.431744
The thermochemical data for the chair transition structure (6-31G*) is displayed below.
Sum of electronic and zero-point Energies= -234.414933 Sum of electronic and thermal Energies= -234.409011 Sum of electronic and thermal Enthalpies= -234.408066 Sum of electronic and thermal Free Energies= -234.443819
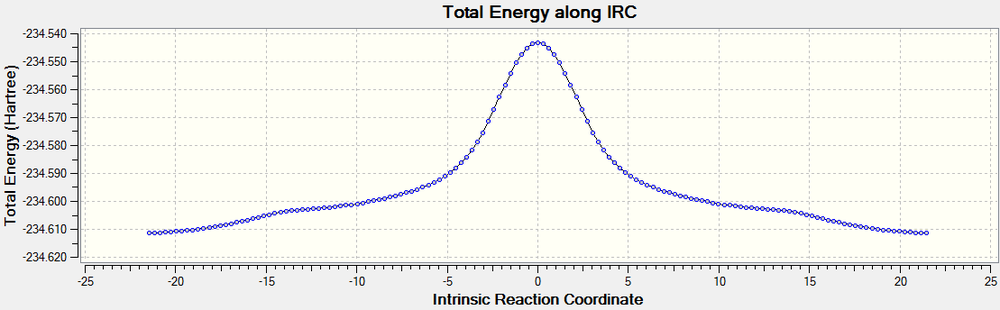
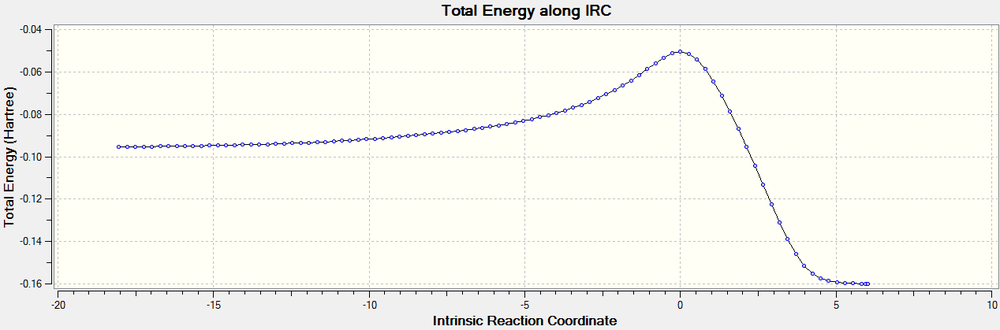
An IRC calculation was then run on each transition state to determine the activation energy. The boat IRC was run with 120 steps and used the 6-31G* basis set. The force constants were set to calculate always. The boat IRC calculation returned 143 conformations, with a minimum being reached for both the transition states and the products. From this the activation energy can be calculated. We can see from the IRC path that the conformation changes before any bonds are broken, then the rearrangement occurs before the molecule changes to the most stable conformation (gauche3, E=-234.6112745 au). The conformation present before the rearrangement is the same one that was created when the boat transition structure was optimised using the QST2 method, with an eclipsed conformation (syn periplanar, E= -234.6028026 au). The activation energy will be calculated using the gauche3 conformation as a reactant.
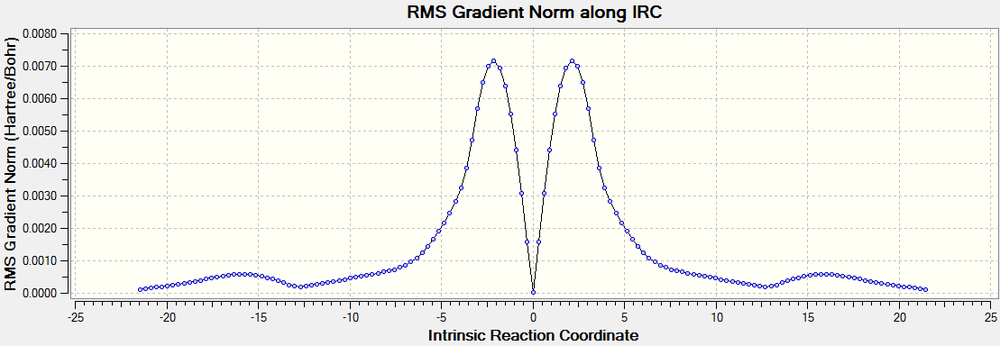
The activation energy of the boat transition cope rearrangement is -0.0681816 au (42.784 kcal/mol). The RMS gradient plot shows the minima caused by the gauche3, syn periplanar and transition state.
File:BOAT 6-31GD IRC HPC LAL.log
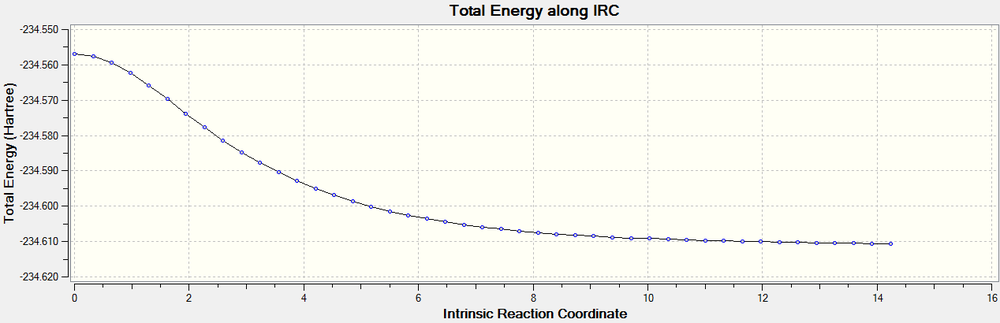
The IRC calculation run for the chair transition state was only run in the forward direction, as it is a symmetrical reaction. It was calculated with the number of steps at 75. The method/basis set was still set to B3LYP/6-31G*. The calculation heralded 45 results and although the IRC path had bottomed out, a minimum had not actually been found. Due to the fact that a minimum was close, the final structure was optimised to a minimum, giving the gauche2 conformer (E=-234.6106850 au). This was the same conformer as was given in the IRC calculation at the HF/3-21G level of theory. This was used to calculate the activation energy (EA= 0.053702 au, 33.698 kcal/mol).
The RMS gradient plot shows the IRC calculation failed to reach a minimum, but it was very close, therefore justifying the minimum calculation.
File:CHAIR IRC 6-31GD HPC OPTMIN2 LAL.LOG File:CHAIR IRC 6-31GD HPC LAL.log
A new IRC calculation (75 steps) was run on the boat transition structure using the HF/3-21G method/basis set. The calculation returned 51 results and gave the syn periplanar conformation as a local minimum, the IRC did not actually reach a proper minimum so the final conformation was optimised to a minimum using the same basis set. The result gave the gauche3 conformation, which is the same result as that of the 6-31G basis set. Like with the 6-31G IRC, the gauche3 conformer was then used to calculate the activation energy. File:BOAT IRC LAL LAL.LOG File:BOAT IRC MIN LAL.LOG.
The data gathered from the IRC run on the chair rearrangement using the HF/3-21G method/basis set was used to calculate the activation energies for this basis set at 298.15K.
| Summary Energies | ||||||
|---|---|---|---|---|---|---|
| HF/3-21G | B3LYP/6-31G* | |||||
| Electronic Energy (au) | Sum of electronic and zero-point energies (au) | Sum of electronic and thermal energies (au) | Electronic Energy (au) | Sum of electronic and zero-point energies (au) | Sum of electronic and thermal energies (au) | |
| Chair TS | -231.619322 | -231.466699 | -231.461341 | -234.556983 | -234.414933 | -234.409011 |
| Boat TS | -231.602802 | -231.450933 | -231.445304 | -234.54309288 | -234.402336 | -234.396002 |
| Hexadiene anti2 | -231.692535 | -231.539539 | -231.532565 | -234.623175 | -234.481083 | -234.473723 |
The data above summarises the energy of the transition states, as well as the energy of one of the low energy conformers of 1,5-hexadiene. as can be seen, the less thorough 3-21G basis set underestimates the energy of the compounds and transition states.
| Activation Energy Summary (kcal/mol) | |||||
|---|---|---|---|---|---|
| Method/Basis Set | HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Experimental |
| Temperature | 0K | 298.15K | 0K | 298.15K | 0K |
| Chair ΔE | 45.70 | 43.536 | 34.06 | 33.698 | 33.5 ± 0.5 |
| Boat ΔE | 55.60 | 56.387 | 41.96 | 42.784 | 44.7 ± 2.0 |
Values obtained from Appendix 1 in italic.
As can be seen from the table above, the activation energies calculated at the 6-31G* level of theory are closer to the experimental values, the lower level of theory seems to overestimate the activation energy of the rearrangement. The two values for the 6-31G* basis set at 298.15K are within the experimental range for the activation energies. The reported experimental values for activation energy at 0K are all less than that at a higher temperatures, which implies that the rearrangement is a thermal process; the higher the temperature, the lower the activation energy. It is worth noting that the selectivity of this particular rearrangement does not matter as it is a symmetric process, but could potentially be an issue in a non-symmetric process so as to achieve the desired product.
Diels-Alder Exercise
Introduction
The diels alder reaction is a cencerted cycloaddtion reaction between a diene and a dieneophile. The reaction is a [4+2] pericyclic reaction, meaning 4e are contributed from the diene and 2e are contributed from the dieneophile.
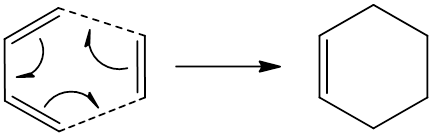
When a diels alder reaction takes place, there is an interaction between the HOMO of one reactant and the LUMO of another. Using the same example as above, if we take the HOMO of butadiene and the LUMO of ethene we can see the LCAO approximation.
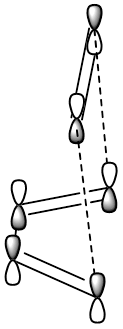
In order for a HOMO-LUMO interaction to be allowed, the two orbitals must have different symmetries to the plane of symmetry in the TS. For example, the LUMO of ethene is antisymmetric, whereas the HOMO of cyclohexadiene is symmetric to the plane. This means the HOMO-LUMO interaction is allowed.
All the calculations in the following section were performed using the Self-empirical/AM1 method, as any of the previous methods used would take too long and have too complex a basis set.
Optimising Butadiene
Cis-butadiene was optimised using the AM1 method. The calculation gave an energy of 0.0487972 au. The HOMO and LUMO were visualised. The HOMO is antisymmetric with regards to the plane and the LUMO is symmetric with regards to the plane, this is shown in the diagram below. This means for an interaction to be allowed with another molecule, that molecule must have an antisymmetric LUMO and a symmetric HOMO with regards to the plane. The .log file can be found here File:BUTADIENE OPT AM1 LAL.LOG
| Butadiene HOMO/LUMO | |
|---|---|
| HOMO | LUMO |
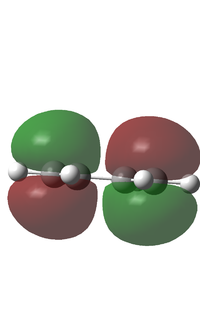 |
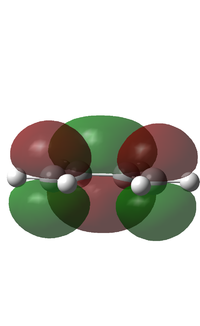
|
| Antisymmetric | Symmetric |
Optimising the Cyclohexene Transition State
A template of bicyclo-2,2,2-octane was used to create a guess for the cyclohexadiene transition state. Part of the molecule was deleted to leave a butene fragment and an ethene fragment. The guess structure was arranged so that the two molecules had the largest pi overlap possible. The two pairs of bonding carbons were arranged to be 2.2 Å apart. The optimisation and frequency were calculated using the AM1 method. The TS was optimised to TS(berny), with the keyword opt=noeigen.
The transition state optimisation was not originally successful, as the optimisation was run to a minimum, giving a chair-like structure with 5 dissociated hydrogens. After this was rectified the optimisation was run successfully. This gave a transition state which was proven by the presence of an imaginary frequency at -956.2cm-1.
As is shown in the above table, the HOMO is antisymmetric relative to the plane of symmetry, whereas the LUMO is symmetric. This is the same as cis-butadiene.
Using the "symmetrize" function, the TS was indicated to have Cs symmetry. The transition structure below shows the measured bond lengths and the theoretical bond lengths of the final structure in brackets.
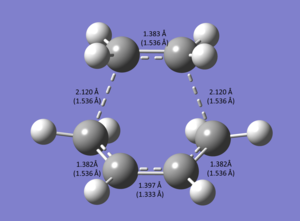
The partially formed C-C bond formed by a breaking of the sp2 C=C bond in ethene has a length of 1.383 Å. This is much lower than a literature sp3 C-C bond (1.536 Å[2]), and therefore still contains a lot of p-character. In addition, the partially formed C=C bond in the hexadiene has a bond length of 1.397 Å. This is longer than the literature C=C sp2 bond (1.333 Å[3]), indicating an increased s character than is in the final molecule. As the Van der Waals radius of carbon is 1.7 Å[4], we would expect a C-C bond to be around 3.4 Å, this shows the increased attraction caused by the sp2 bond. The single bond is longer than this and the double bond is slightly less than this. It is expected that the sp2 bond will be shorter than the sp3 hybrid bonds. This is shown in the transition state, with the C=C bonds that are breaking being slightly longer than those being made. From this observation we can conclude that the transition state contains bonds that are mainly delicalised and almost halfway formed. electron density is in the process of being transferred between the terminal carbon atoms that will form σ C-C bonds.
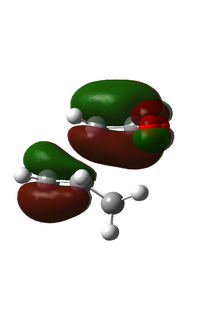
If we look at the HOMO and LUMO for ethene we will see that the HOMO is symmetric and the LUMO is antisymmetric. This is the opposite to cis-butadiene, meaning that the two molecules are allowed to interact.
| Cyclohexene TS HOMO/LUMO | ||
|---|---|---|
| Ethene | Cis-Butadiene | |
| HOMO |  |

|
| Symmetric | Antisymmetric | |
| LUMO | 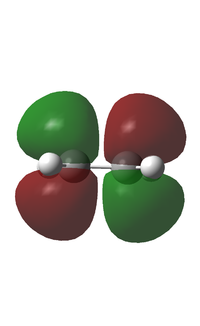 |

|
| Antisymmetric | Symmetric | |
We can also see from the HOMO and LUMO of these two molecules that there is a large overlap of electron density. This means they are likely to interact, which is shown in the HOMO of the transition state by large delocalised orbitals in the TS HOMO.
| Cyclohexene TS HOMO/LUMO | |||
|---|---|---|---|
| HOMO |  |
 |
Antisymmetric |
| LUMO |  |
 |
Symmetric |
The fact that this is a transition state was proven by the existence of an imaginary frequency (-956.2 cm-1). This vibration (below) is a synchronised formation of bonds. It looks like a stretch along the bonds that are to be formed.
| Cyclohexene TS |
|---|
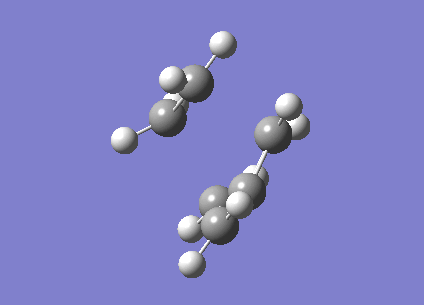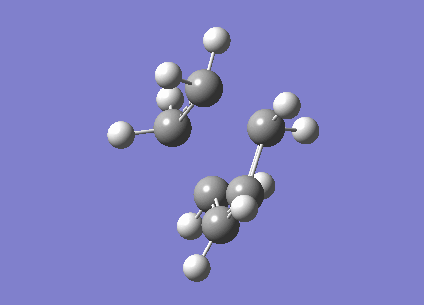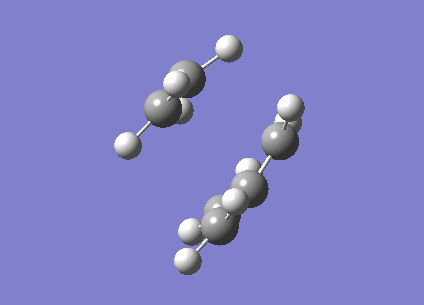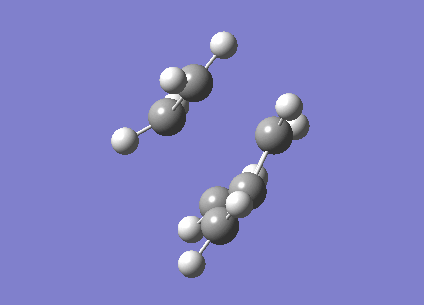
|
In comparison, the next lowest positive frequency (147.3 cm-1) appears to be a bend in the C=C in ethene, but the cis-butadiene is rotating in the opposite direction. This is a very low peak for an IR spectrum, and there are more similar peaks at very low frequencies. These are interactions between the two molecules, but due to the low bond length of the partially formed C-C bonds, the strength of the bonds are much lower than they usually would be, which affects the frequency of vibrations.
File:CYCLOHEXENE TS OPTFREQ BERNY AM1 2 LAL.LOG File:ETHENE OPT LAL.LOG
Maelic Anhydride and Cyclohexadiene
The following calculations were run with the self-emperical method and AM1 basis set.
Optimisations
Cyclohexadiene and maelic anhydride were optimised separately before combining them to form guess transition states for the endo and exo products of the cycloaddition. The two pairs of bonding carbons were frozen at a distance of approximately 2.2 Å. The structure was then optimised to a minimum. This gave the structures below.
| Diels-Alder TS Optimisation Minimum | |
|---|---|
| Endo | Exo |
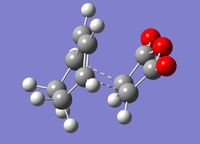 |
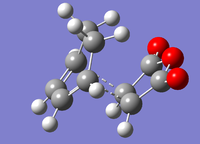
|
| File:DIELS ALDER ENDO OPTFREQ AM4 LAL.LOG | File:DIELS ALDER EXO OPT1 LAL.LOG |
The coordinates were then set to derivative and the hessian matrix was then used to optimise the transition state. An opt+freq calculation was run, optimising to a TS(berny).
The two transition states were modeled successfully, giving imaginary frequencies of -806.9 cm-1 and -811.9 cm-1 for the endo and exo structures respectively.
TS Analysis
| Diels-Alder Transition States | ||
|---|---|---|
| Endo | Exo | |
| Energy (au) | -0.0515045 | -0.0504191 |
| Energy (kcal/mol) | -32.320 | -31.638 |
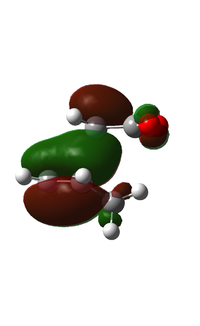
| TS Vibration |  |
|
| HOMO |  |
|
| HOMO | 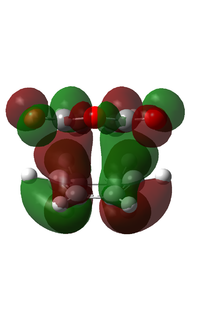 |
|
| HOMO Energy (au) | -0.33189 | -0.34275 |
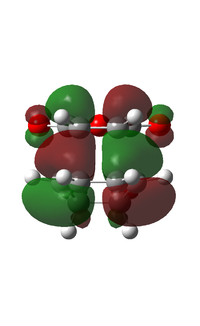
| LUMO |  |
|
| LUMO | 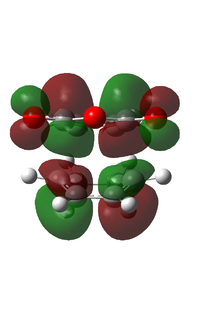 |

|
| LUMO Energy (au) | -0.18913 | -0.04045 |
| File | File:DIELS ALDER ENDO OPTFREQ AM4 DERIV2 LAL.LOG | File:DIELS ALDER EXO OPTFRQ1 LAL.LOG |
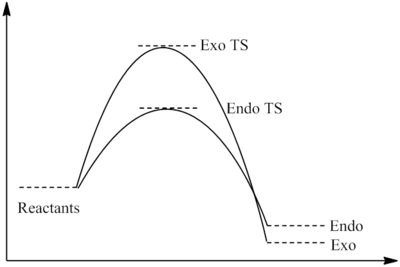
As can be seen from the above table, the energy of the endo TS is lower than that of the exo TS. As we know the reaction is under kinetic control, which means the product with the lowest energy transition state will be formed first. Judging by the data calculated, this will be the endo product. Literature confirms this, and experimentally the product formed will be 99% endo [5]. The transition state vibration shows that the bonds are formed simulaneously, this implies that there is only one step in the reaction and no intermediate. From this we can draw the reaction profile below.
We can see from the transition state illustrations that the endo TS is much less sterically hindered than the exo form. This is because in the exo form the bulk of the maelic anhydride is situated on top of the CH2 groups in cyclohexadiene. As this is the non-planar part of the molecule, steric hindrance ooccrus, raising the energy of the TS. This is shown by the bond distance achievable by the newly bonding carbon atoms. In the exo TS the inter atom distance is 2.171 Å, whereas the less hindered endo TS has an inter atom distance of 2.163Å.
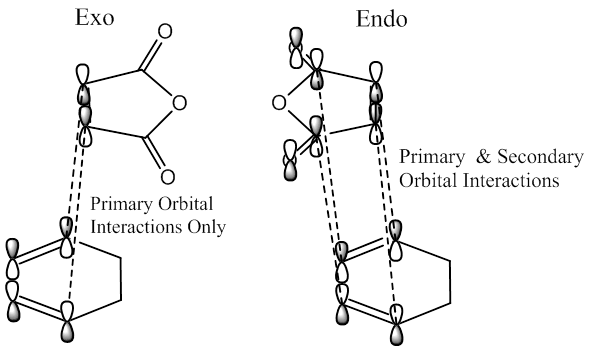
Secondary Orbital Effect
The reason the endo product is favoured can be explained by the secondary orbital effect, this is a phenomenon only experienced in endo transition states. As we know, the diels alder cycloaddition is caused by interaction between π orbitals. In this case the π orbitals of the C=C bond in maelic anhydride and the π orbitals of one of the carbons in each C=C bonds in cyclohexadiene. This is the primary orbital interaction, and is present in both transition states. However, in the endo TS, there is an extra interaction made possible by the orientation of the maelic anhydride, this makes it possible for an in phase interaction between the π* C=O orbitals on the carbons and the other
HOMO/LUMO Analysis
Upon examination of the HOMO for the two transition states, we can see the electron interaction between the two molecules. This is shown by a large delocalised orbital between the bonding carbons. We can also see the secondary orbital overlap effect at work here. This is shown by an in phase relation between the MO between the C(O)-O-C(O) atoms and the large delocalised orbital between the bonding atoms. This is situated on the endo transition state. This is also shown by the increased size of the large delocalised orbital, as well as the increased size of the carbonyl π* MO.
Activation Energy Analysis
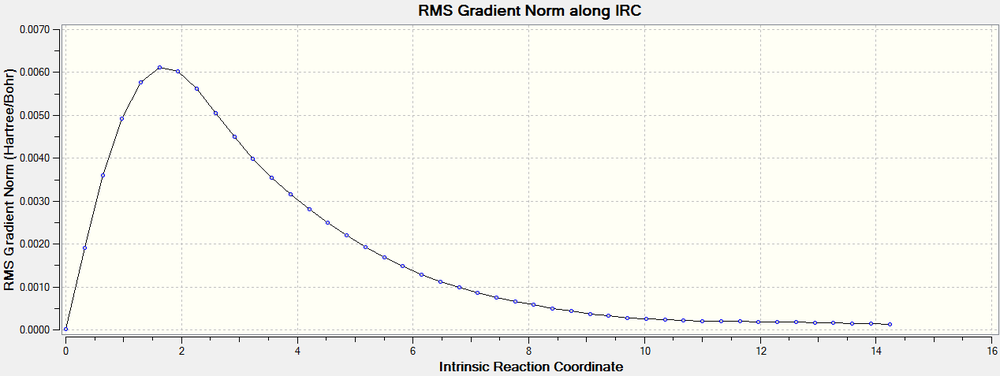
An IRC calculation was run on the exo transition state in both directions. The force constants were set to calculate always and 120 points were to be calculated. The result returned 93 states, which are shown below. The energy of each of these states were plotted against the reaction coordinate to give the reaction profile. From this the activation energy can be calculated by finding the energy difference between the transition state and the sum of the two reactant energies. In this case the activation energy is (-0.05041907-(-0.09537219)= 0.04495312 au (28.21 kcal/mol).
Some final, additional proof that a transition state has been reached is shown below. The graph shows a minimum in the RMS gradient at the same point as the transition state. This indicates that the structure achieved is indeed a transition state.
The same IRC calculation was run on the endo transition state, with similar results. As can be seen by the reaction profile below. The activation energy of the endo reaction was therefore calculated to be 0.04286216 au (26.90 kcal/mol).
The RMS gradient plot below also offers proof that the transition state modeled is in fact a transition state for the diels alder reaction with the presence of a minimum.
The endo and exo products were both optimised and underwent frequency calculation (semi-empirical/AM1) in order to work out their energy data. File:EXO PRODUCT FREQ LAL.LOG File:EXO PRODUCT LAL.LOG File:ENDO OPTFREQ PRODUCT LAL.LOG.
| Summary energies | |||
|---|---|---|---|
| Electronic Energy (au) | Sum of electronic and zero-point energies (au) | Sum of electronic and thermal energies (au) | |
| Endo TS | -0.051505 | 0.133495 | 0.143686 |
| Exo TS | -0.050419 | 0.134877 | 0.144881 |
| Endo Product | -0.160171 | 0.031464 | 0.040463 |
| Exo Product | -0.159909 | 0.031704 | 0.040683 |
These Energies may seem strange, but this will be due to the method used (AM1). The basis set is not accurate enought to achieve good enough accuracy in energy calculations.
| Summary of Activation energies (298.15K, kcal/mol) | |
|---|---|
| Endo | 26.90 |
| Exo | 28.21 |
Using summary above we can definitively see the reason why the endo product is favored so highly over the exo product. The activation energy is lower (by 1.31 Å), meaning this transition state is reached first. As we know the reaction is under kinetic control, which means that the product with the lowest energy transition state is predominantly formed.
The reason for the endo TS being a lower energy than the exo, as discussed previously is the presence of the secondary orbital overlap effect. This effect is the stabilisation of the endo product through interaction between an in phase interaction between the π* C=O orbital on the maelic anhydride and the π orbital on one of the carbon atoms on the cyclohexadiene. This was shown by LCAO as well as the visualised HOMO and LUMO of the TS.
Exo TS thermodynamic data:
Sum of electronic and zero-point Energies= 0.134877 Sum of electronic and thermal Energies= 0.144881 Sum of electronic and thermal Enthalpies= 0.145825 Sum of electronic and thermal Free Energies= 0.099105
Endo TS thermodynamic data:
Sum of electronic and zero-point Energies= 0.133495 Sum of electronic and thermal Energies= 0.143686 Sum of electronic and thermal Enthalpies= 0.144630 Sum of electronic and thermal Free Energies= 0.097346
Conclusions
Its has been shown that modelling programs such a gaussview and gaussian can be used to not only optimised molecules to various levels of theory, but can be used to gain an accurate insight into the bond breaking and forming of a reaction. It has also shown the structure of transition states and wheher the bond forming is concerted or not. The HOMO and LUMO of transition states have been visualised and examined, proving the theory behind the diels alder reactions, such as allowed HOMO-LUMO interactions as well as symmetry properties. In addition to all this, the activation energies of reactions ans rearrangements have been calculated and used to prove the regioselectivity of the diels alder reaction between maelic anhydride and cyclohexadiene.
<references> [2] [3] [4] [1] [5]
- ↑ 1.0 1.1 Arthur C. Cope; et al.; J. Am. Chem. Soc. 1940, 62, 441.
- ↑ 2.0 2.1 S. Hedberg, J. Am. Chem. Soc, 1951, 73, 1486
- ↑ 3.0 3.1 S. Dowling J. Chem. Phys, 1959, 31, 400-403
- ↑ 4.0 4.1 A. Bondi, J. Chem. Phys., 1964, 68, 441-451
- ↑ 5.0 5.1 R. Gleiter, M. C. Bohm, Pure & Appl.Chem., 1983, 55, 237—244