Rep:Mod:ll4010
Introduction to Gaussian
Optimising a BH3 Molecule
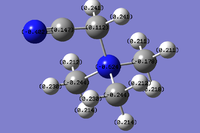
The BH3 molecule was first drawn in gaussview, and the bond lengths changed to 1.5 angstroms. The optimisation was run with the B3LYP method and the 3-21G basis set. The optimised B-H bond length was given to be 1.19 angstroms, with the bond angle given as 120o.
The data for the BH3 molecule optimisation is below. The optimisation file can be found at File:BH3OPT LL.LOG.
As shown below, Gaussian has analysed the energy of the molecule at various nuclei positions, and modeled the forces to converge at the bond lengths and angles at the values in the table at the bottom.
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1935 -DE/DX = 0.0004 !
! R2 R(1,3) 1.1935 -DE/DX = 0.0004 !
! R3 R(1,4) 1.1935 -DE/DX = 0.0004 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
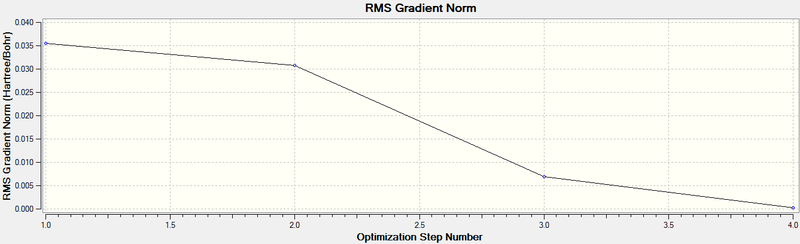
The optimisation plots were then analysed, the first plot, that of total energy versus optimisation step number shows a steady downward slope up to the third optimisation step, followed by a very shallow decrease in energy between the third and fourth step. This makes sense as the program will be decreasing the energy rapidly at first, and as soon as the energy begins to equilibrate, the energy decrease will lessen.

The second plot shows the forces on the molecule (F=dE/dR) against the optimisation step number. This decreases to close to zero at step 4, as the optimisation process works to find an equilibrium between the forces and therefore minimise the energy.
Using Improved Basis Sets
The function was run again, on the previous optimised structure, with a new basis set (6-31g(d,p)). This is found here File:BH3OPT 631G DP LAL.LOG. The new basis set gave a B-H bond length of 1.19 Å and a H-B-H bond angle of 120o.
The difference in energies from the two basis sets (-26.6172224 au and -26.4622634 au) is 0.1549590 au. This is a large energy gap, and so shows that using different basis sets makes it impossible to compare the two results. It is also equal to around 400kJ/mol, indicating the accuracy of reporting required. If kJ/mol is reported to 2dp, then au must be reported to 7dp. The final optimisation data is displayed below.
Item Value Threshold Converged?
Maximum Force 0.000422 0.000450 YES
RMS Force 0.000276 0.000300 YES
Maximum Displacement 0.001666 0.001800 YES
RMS Displacement 0.001091 0.001200 YES
Predicted change in Energy=-1.132958D-06
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.192 -DE/DX = 0.0004 !
! R2 R(1,3) 1.192 -DE/DX = 0.0004 !
! R3 R(1,4) 1.192 -DE/DX = 0.0004 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Using Pseudo Potentials and new Basis Sets
TlBr3 Optimisation
An optimisation calculation was submitted to the Imperial College HPC for TlBr3 with the basis set LanL2DZ. The Tl-Br bond length was found to be 2.65 Å with a Br-Tl-Br bond angle of 120°. This bond length value compares to that found in the literature, these are 2.55 Å[1], 2.495 Å [2] and 2.478 Å [3]. These are all smaller than that calculated by the optimisation, however it is quite close to the literature values.
The confirmation that the optimisation was successful, and that the forces and displacement converged is below. DOI:10042/21587 .
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.083976D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 2.651 -DE/DX = 0.0 !
! R2 R(1,3) 2.651 -DE/DX = 0.0 !
! R3 R(1,4) 2.651 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Mixing Pseudo Potentials and Basis Sets
BBr<sub\>3 Optimisation
The optimisation for BBr<sub\>3 requires a mixture of basis sets and pseudo potentials. The low electron count boron is governed by the basis set, and the heavy Br atoms are governed by a pseudo potential. The optimised bond lengths are 1.93 Å and the Br-B-Br bond angles are 120°. This ties in with the reported point group of D3h.
Item Value Threshold Converged?
Maximum Force 0.000246 0.000450 YES
RMS Force 0.000161 0.000300 YES
Maximum Displacement 0.001151 0.001800 YES
RMS Displacement 0.000754 0.001200 YES
Predicted change in Energy=-4.716288D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.9333 -DE/DX = 0.0002 !
! R2 R(1,3) 1.9333 -DE/DX = 0.0002 !
! R3 R(1,4) 1.9333 -DE/DX = 0.0002 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Analysis of Results
| Bond Lengths | |
|---|---|
| BH3 (B-H) | 1.19 Å |
| BBr3 (B-Br) | 1.93 Å |
| TlBr3 (Tl-Br) | 2.65 Å |
The above table illustrates the bond lengths calculated for the three molecules. The smallest bond length is the B-H bond. This would be because Boron and Hydrogen are hard atoms, with a high charge density, meaning the coulombic forces are larger. This is backed up by the increase in bond length by 0.74 Å when hydrogen is replaced by the much larger, much softer Bromine. Bromine also has a much large atomic radius, meaning that the equilibrium atom distance is larger as well. The hydrogen and bromine are in opposite groups, 1 and 17 respectively, although due to its unique structure, and the fact it only has to fill the 1s orbital, hydrogen can exibit group 17 properties. The relationship between the increase in bond length and the increase in atomic number is 0.021803.
Changing the central atom in the molecule from Boron to Thallium has a large effect on the bond length. This is shown by the increase in bond length by 1.46 Å when the central atom is changed. Boron and thallium are both in group 5,. The relationship between the increase in bond length and the increase in atomic number is 0.019196, this is smaller than that calculated for hydrogen to bromine, as the difference is quite small it may be meaningless, however it does imply that the effect on bond length is greater for a change in ligand than a change in metal centre.
Frequency Analysis
BH3 Frequency Analysis
A frequency calculation was run on the optimised molecule of BH3 using the improved basis set (6-31+G(d,p)). The results summary is shown below.
The log file can be accessed here: File:LUKELOMBARDO BH3 FREQ.LOG. The two low frequency lines are shown below. There are three frequencies outside the ±15 cm-1 from zero range. This would be down to the method used.
Low frequencies --- -69.3869 -68.6537 -68.6535 -0.0054 0.0740 0.1612 Low frequencies --- 1156.1707 1204.2826 1204.2849
| BH3 Vibration Modes | ||||
|---|---|---|---|---|
| Mode # | Vibration Form | Frequency (cm-1) | Intensity | Symmetry |
| 1 | The H atoms all move in the same direction, perpendicular to the plane that the atoms are in. The B atom oscillates in the opposite direction | 1156.17 | 93.9187 | E' |
| 2 | The atoms move in the same plane. 1 H and the central B atom rock slightly in the same direction as each other in the plane of the atom. The other two hydrogen atoms move in a symmetric seesaw direction in the same plane. | 1204 | 13 | E' |
| 3 | All four atoms move in the same plane as the molecule. Two hydrogens oscillate in sync around the B atom. The other oscillates in the opposite direction. | 1204 | 13 | A2' |
| 4 | All H atoms move in and out together in a concerted motion. the B atom is stationary. | 2581 | 0 | Fully symmetric: A1' |
| 5 | One H atom is stationary, the B atom rocks from side to side and the other two B-H bonds stretch asymmetrically. | 2709 | 142 | E' |
| 6 | Two B-H bonds stretch symmetrically, while the other stretches out of time, and more vigorously. The B atom rocks gently in an opposite direction to the asymmetric hydrogen. | 2709 | 142 | E' |
The data above shows the 6 modes of vibration that cause the peaks in the IR spectrum (Below). There are however only 3 peaks in the spectrum. This is partly due to the cat that mode #4 causes no change in dipole moment, making it IR inactive. Modes 2 and 3 also have very similar frequencies. This means they combine together to form the same peak. The same happens for peaks 5 and 6. This effect causes the peaks to be slightly more broad than usual.
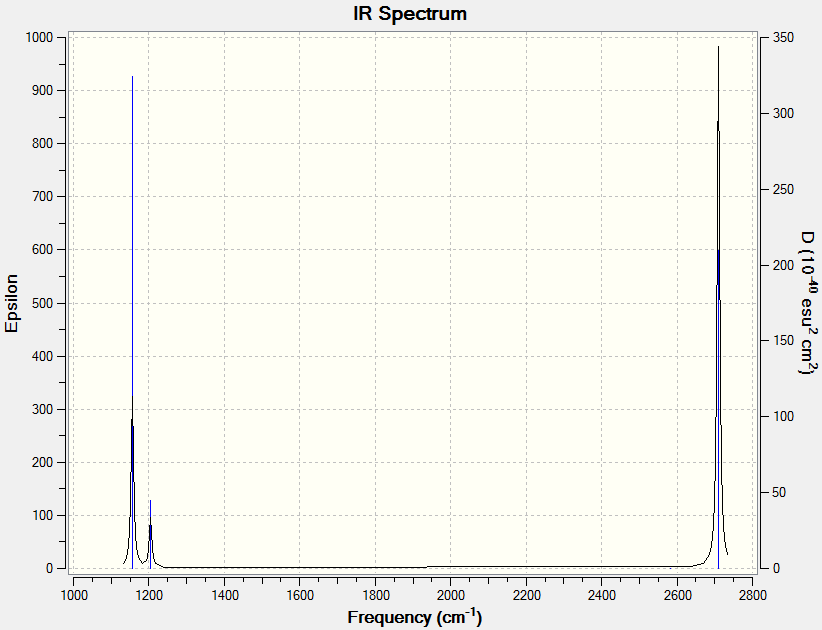
TlBr3 Frequency Analysis
A frequency analysis was run on the HPC with the previously optimised structure. DOI:10042/21695 .
The log file can be accessed here File:TlBr3 Frequency LAL.log. The two low frequency lines are shown below, these lie within the allowed ±15 cm-1 range.
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367 Low frequencies --- 46.4289 46.4292 52.1449
| TlBr3 Vibration Modes | ||||
|---|---|---|---|---|
| Mode # | Vibration Form | Frequency (cm-1) | Intensity | Symmetry |
| 1 | The atoms move in the same plane. 1 Br and the central Tl atom rock slightly in the same direction as each other in the plane of the atom. The other two bromine atoms move in a symmetric seesaw direction in the same plane. | 46 | 4 | A2 |
| 2 | All four atoms move in the same plane as the molecule. Two bromines oscillate in sync around the Tl atom. The other oscillates in the opposite direction. | 46 | 4 | E' |
| 3 | The Br atoms all move in the same direction, perpendicular to the plane that the atoms are in. The Tl atom oscillates in the opposite direction. | 52 | 5.8466 | E' |
| 4 | All Br atoms move in and out together in a concerted motion. The Tl atom is stationary. | 165 | 0 | Fully symmetric: A1' |
| 5 | One Br atom is stationary, the Tl atom rocks from side to side and the other two Tl-Br bonds stretch asymmetrically. | 211 | 25 | E' |
| 6 | Two Tl-Br bonds stretch symmetrically, while the other stretches out of time, and more vigorously. The Tl atom rocks gently in an opposite direction to the asymmetric bromine. | 211 | 25 | E' |

| Vibrational Frequency (cm-1) | ||
|---|---|---|
| TlBr3 | BH3 | |
| 1 | 46 | 1157 |
| 2 | 46 | 1204 |
| 3 | 52 | 1204 |
| 4 | 165 | 258 |
| 5 | 211 | 2710 |
| 6 | 211 | 2710 |
The table above shows vibration frequencies for TlBr3 and BH3. These values are very far apart, this is most probably down to the strength of the bonds. The stronger the bonds, the more energy required to vibrate them. The B-H bond is a much stronger than the Tl-Br bond, this is because of the larger orbital overlap caused by the smaller atomic radius(and subsequently shorter bond length) in the B-H bond.
The order of the modes also changes with thallium tribromide. In the boron compound, the lowest frequency corresponds to an out of plane saw motion, however this bend is the third highest energy in the thallium compound. The stretches however are in the same location.
The two IR spectra are a similar shape, with the thalium spectrum occuring at a much lower intensity. The thallium spectrum also has two signals so close together they combine into one broad peak. The boron compound however has two distinct peaks here. The thallium compound is also a much lower intensity, this may be trivial though, as it may not be possible to compare intensities between different compounds.
MO Calculation
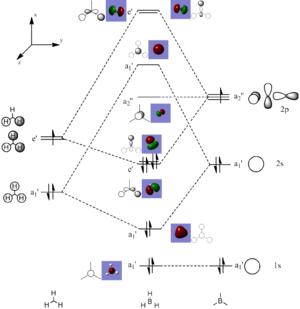
An MO calculation was run on the optimised checkpoint file of BH3. The 8 lowest molecular orbitals were visualised and added to the diagram (right). These were also summarised in the table below, next to their LCAO approximations. As we can see, the qualitative LCAO approximations are very close to the quantitative orbitals, the only thing that is not represented well is the actual size of the orbitals. This is shown by orbital 6, the antibonding orbital of the boron is so diffuse it engulfs the molecule, whereas the LCAO approximation shows orbital phases.
| MOs Vs LCAO | |||
|---|---|---|---|
| Energy Level | MOs | LCAOs | |
| 1 |  |
 | |
| 2 |  |
 | |
| 3 |  |
 | |
| 4 |  |
 | |
| 5 |  |
 | |
| 6 |  |
 | |
| 7 |  |
 | |
| 8 |  |
 | |
NH3 Analysis
Optimisation
An optimisation was run on a molecule of NH3 with the method RB3LYP and basis set 6-31+G(d,p).
The N-H bond angle was found to be optimised at 1.00 Å, with a H-N-H angle of 108.9°. The optimisation converged as shown below. The file can be found at File:NH3 OPT DFT LAL.LOG.
Item Value Threshold Converged?
Maximum Force 0.000036 0.000450 YES
RMS Force 0.000031 0.000300 YES
Maximum Displacement 0.000756 0.001800 YES
RMS Displacement 0.000361 0.001200 YES
Predicted change in Energy=-4.164926D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.0158 -DE/DX = 0.0 !
! R2 R(1,3) 1.0158 -DE/DX = 0.0 !
! R3 R(1,4) 1.0158 -DE/DX = 0.0 !
! A1 A(2,1,3) 108.0763 -DE/DX = 0.0 !
! A2 A(2,1,4) 108.0763 -DE/DX = 0.0 !
! A3 A(3,1,4) 108.0763 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -116.7354 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Vibrational Analysis
Frequency analysis was then run, using the method opt+freq, and the keywords integral=grid=ultrafine. This ensured the low frequencies were well within the ±15 cm-1 acceptable range. The low frequencies are shown below. The file can be found here File:NH3 FREQ DFT LAL.LOG.
Low frequencies --- -7.4398 -0.2086 -0.1379 -0.0016 1.6522 1.6853 Low frequencies --- 998.5929 1673.3999 1673.4000
| NH3 Vibration Modes | ||||
|---|---|---|---|---|
| Mode # | Vibration Form | Frequency (cm-1) | Intensity | Symmetry |
| 1 | The H atoms all bend in the same direction, with the nitrogen moving in the opposite direction. The motion moves along the C3 axis, deviating slightly | 999 | 241 | E' |
| 2 | Two H atoms bend one way around the nitrogen, with the other rotating the other way. The nitrogen rocks side to side in time. | 1673 | 29 | E' |
| 3 | Two hydrogens bend towards each other, with the other, with the other bending upwards when the first two come together. The nitrogen rocks slightly, in time. | 1673 | 29 | A2' |
| 4 | All three hydrogen atoms stretch at the same time, with the nitrogen rocking along the C3 axis. | 3485 | 3 | Fully symmetric: A1' |
| 5 | One H stays stationary, while the other two stretch asymmetrically. The Nitrogen rocks gently with the stretching. | 3629 | 4 | E' |
| 6 | Two hydrogens stretch symmetrically, while the other stretches asymmetrically and more vigorously. The nitrogen rocks gently in time. | 3629 | 4 | E' |
The IR spectrum below shows the absorption bands corresponding to the vibrations of ammonia. It shows three main peaks, corresponding to 5 absorptions. There is also a stretch that does not show up on the spectrum. This corresponds to a symmetric stretch. Although in the trigonal planar equivalent (BH3), a small change in dipole moment, via movement of the nitrogen atom, causes this stretch to be IR active.

Population Analysis
Molecular orbital analysis was then undertaken on the frequency checkpoint file. The molecular orbitals (shown below) are similar to that of BH3, however due to its tetrahedral shape they differ. The .log file can be found at File:NH3 MO DFT LAL.LOG. DOI:10042/21755 .
NBO Analysis
The charge data analysis showed that the NBO charge for the hydrogen atoms was +0.394, whereas the charge for the Nitrogen was -1.182. The charge distribution image (-1.182 to +1.182) is shown below.
From the NBO analysis we can see that the hybridisation of the N-H bonds is 26.41% s-character and 73.52% p-character for N and 100%s-character for H. In all of the N-H bonds, the nitrogen contributes 69.74% of the electron density to the bond, whereas the hydrogen atoms contribute 30.26% each. The fifth orbital contains the nitrogen lone pair, which has 20.68% s-character and 79.24% p-character.
This implies that NH3 has 4, sp3 hybridised orbitals.
NH3BH3 Analysis
Optimisation
An optimisation was run on NH3BH3 on the HPC, the data is shown below. All forces converged. The optimisation gave a B-N bond length of 1.67015 Å, it also gave a N-H bond length of 1.01871 Å and a B-H bond length of 1.21172 Å. The optimisation also reported bond angles of 107.87°(H-N-H), 111.03°(H-N-B), 104.70°(N-B-H) and 113.79°(H-B-H). The file can be found here; File:NH3BH3 OPT 6-31GDP LAL.log. DOI:10042/21802 .
Item Value Threshold Converged?
Maximum Force 0.000117 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.000481 0.001800 YES
RMS Displacement 0.000240 0.001200 YES
Predicted change in Energy=-1.105960D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,4) 1.2117 -DE/DX = -0.0001 !
! R2 R(2,4) 1.2117 -DE/DX = -0.0001 !
! R3 R(3,4) 1.2117 -DE/DX = -0.0001 !
! R4 R(4,8) 1.6702 -DE/DX = 0.0 !
! R5 R(5,8) 1.0187 -DE/DX = -0.0001 !
! R6 R(6,8) 1.0187 -DE/DX = -0.0001 !
! R7 R(7,8) 1.0187 -DE/DX = -0.0001 !
! A1 A(1,4,2) 113.7955 -DE/DX = 0.0 !
! A2 A(1,4,3) 113.7889 -DE/DX = 0.0 !
! A3 A(1,4,8) 104.7009 -DE/DX = 0.0 !
! A4 A(2,4,3) 113.7861 -DE/DX = 0.0 !
! A5 A(2,4,8) 104.7029 -DE/DX = 0.0 !
! A6 A(3,4,8) 104.7012 -DE/DX = 0.0 !
! A7 A(4,8,5) 111.0312 -DE/DX = 0.0 !
! A8 A(4,8,6) 111.0252 -DE/DX = 0.0 !
! A9 A(4,8,7) 111.0331 -DE/DX = 0.0 !
! A10 A(5,8,6) 107.8728 -DE/DX = 0.0 !
! A11 A(5,8,7) 107.8661 -DE/DX = 0.0 !
! A12 A(6,8,7) 107.867 -DE/DX = 0.0 !
! D1 D(1,4,8,5) 59.944 -DE/DX = 0.0 !
! D2 D(1,4,8,6) 179.9471 -DE/DX = 0.0 !
! D3 D(1,4,8,7) -60.0558 -DE/DX = 0.0 !
! D4 D(2,4,8,5) 179.9503 -DE/DX = 0.0 !
! D5 D(2,4,8,6) -60.0466 -DE/DX = 0.0 !
! D6 D(2,4,8,7) 59.9504 -DE/DX = 0.0 !
! D7 D(3,4,8,5) -60.054 -DE/DX = 0.0 !
! D8 D(3,4,8,6) 59.9491 -DE/DX = 0.0 !
! D9 D(3,4,8,7) 179.9461 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Frequency Analysis
A frequency analysis was also carried out using the same basis set. The low frequencies shown below are all positive, meaning a minimum is present. The six frequencies above this show that the method used was good. The file can be found here; File:NH3BH3 FREQ 6-31GDP LAL.LOG. DOI:10042/21811 .
Low frequencies --- 0.0006 0.0009 0.0012 11.7694 18.6689 32.7270 Low frequencies --- 276.7892 629.8609 643.2565
Energy Comparisons
| Energy Summary | |
|---|---|
| Molecule | Energy (au) |
| NH3 | -56.5669853 |
| BH3 | -26.6172224 |
| NH3BH3 | -83.2307183 |
The energies of BH3, NH3 and NH3BH3 are summarised above. From this the association energy of combining a molecule of NH3 and BH3:
ΔE = E(NH3BH3)- E(NH3)- E(BH3)
Therefore ΔE = -0.0465106 au
This then needs to be converted to kJ/mol by using the relationship 1 au = 2625.54 kJ/mol. This gives us an energy change of:
ΔE = -122.11 kJ/mol
Mini Project: Ionic Liquids as Designer Solvents
Ionic Liquids Background
Ionic liquids are a new and fascinating substance, they are a liquid consisting of just ions. While most ionic systems can be molten, ionic liquids are liquid at room temperature. They have a wide variety of uses, as designer solvents, lubricants and catalysts. Recent studies have shown that using mixtures of ionic solvents will change properties such as density, thermal stability and many others[4], making it possible to design particular ionic solvents for certain purposes.
Ionic liquids typically consist of a bulky R-group cation and an inorganic anion (e.g halides. In this project various tetraalkyl cations will be modeled to investigate the distribution of charge and their suitability for use as an ionic liquid.
[N(CH3)4]+ Analysis
Optimisation
An optimisation of the [N(CH3)4]+ ion was run using the full basis set (6-31G+(d,p)) on the department HPC. The optimisation converged (see below). The optimisation gave bond lengths of 1.51Å (C-N)and 1.09Å (C-H). It also gave bond angles of 110.0° (H-C-H), 109.5° (C-N-C) and 108.9° (H-C-N). These correspond to a fully tetrahedral geometry. File:-N(CH3)4- OPT LAL.log. DOI:10042/21818
Item Value Threshold Converged?
Maximum Force 0.000135 0.000450 YES
RMS Force 0.000029 0.000300 YES
Maximum Displacement 0.001136 0.001800 YES
RMS Displacement 0.000308 0.001200 YES
Predicted change in Energy=-1.580841D-07
Optimization completed.
-- Stationary point found.
Frequency
The frequency calculations run on the nitrogen ion show that the optimised molecule has formed a minimum. This is shown by the lack of negative frequencies in the log file. This can be found here: File:-N(CH3)4- FREQ LAL.log. DOI:10042/21821 .
Low frequencies --- 0.0004 0.0007 0.0008 3.2366 9.3060 10.1887 Low frequencies --- 180.3693 289.1791 289.9410
The vibrational data for the nitrogen ion shows 45 vibrational modes. 17 of these are IR inactive, meaning there is no change in dipole moment. These vibrations are all symmetrical, with the nitrogen atom staying stationary at all times. There are a further 7 modes where the intensity is very small (Intensity= 0.00 2dp) and the nitrogen atom seems to not move in all of the animations, as well ast the modes being symmetrical. We can therefore assume that these are also IR inactive. Of the remaining 21 vibrational modes, only 9 have an intensity of more than 2, and so will most likely be the most prominent on the IR spectrum. Finally, these 9 modes fall into three bands, each at a different frequency. Each band contains three vibrational modes of very similar energy. These three bands correspond to the three main peaks in the IR spectrum (summarised in table).
![An IR spectrum of [N(CH3)4]+](/images/d/d9/-N%28CH3%294%29-_IRLAL.png)
MOs
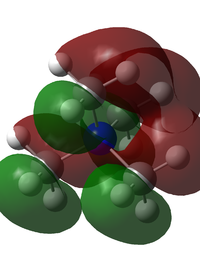
The MO analysis for [N(CH3)4]+ produced 21 occupied orbitals. These were visualised and a range were selected and added to the table below. The core orbitals were not included in the analysis, these were identified as being an order of magnitude lower in energy (approx. -10 au) than the lowest non-core orbital. The first MO reported in the table is the lowest non-core orbital in the ion, it is the 6th highest MO in the ion. As the picture shows, it portrays a strong bonding interaction, due to there being one phase overall. This also means that there are through space interactions. The level of delocalisation is high, due to the MO engulfing almost the whole molecule, there being only one phase and there being no nodes.
The second MO shown is the 14th highest MO, and experiences mixed bonding character. There are 2 planar nodes present, indication a small amount of antibonding character. Each phase present experiences through space interactions, this means there are 4 delocalised areas. The energy is -0.62386 au.
The third MO is the HOMO orbital for the ion, it experiences quite strong interactions, with quite a low energy. The orbital interactions are predominantly bonding, which is shown by large phases and a lot of through space interactions. There are 3 nodes in this MO, and a small amount of delocalisation.
The fourth MO is the LUMO, this is the first unoccupied orbital and exhibits weak AO interactions. This is shown by the carbon atoms alone being surrounded by a surface of opposite phase to the rest of the ion. The nitrogen atom also exhibits this. This is shown by the energy of the orbital as well, changing from -0.58080 au in the HOMO (MO3) to -0.15690 au in this, the LUMO. There is not much delocalisation in this MO either.
The final MO shown here is the 41st energy level, and has a much higher energy than the HOMO (0.01876 au). There are many nodes present and it it mostly antibonding. There is limited through space bonding and almost no delocalisation.
File:-N(CH3)4- FREQ1 MO LAL.log. DOI:10042/21833
NBOs
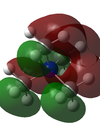
The charge data analysis showed that the hydrogen atoms in the [N(CH3)4]+ ion carry a charge of +0.273. This compares to the more negative carbon atoms, with a charge of -0.485. Interestingly, The nitrogen atom has a higher charge of -0.341, most likely due to the positive charge on the ion mostly being located here. The charge distribution is shown graphically, with red being negative, and green being positive (range= -1 to 1).
The log file shows that each C-H bond shares the electron density in a 63.67:36.33 ratio (C:H). The carbon hybrid orbitals also show 26.75% s-character and 73.20% p-character. This indicates an sp3 hybridisation, which coincides with the tetrahedral shape.
The C-N bonds all have an electron density ratio of 33.21:66.79 (C:N). This would be down to the higher electronegativity of nitrogen. The nitrogen hybridisation data shows signs of sp3 hybridisation (25% s-character.
The orbital summary shows that all C-H bonds have the same energy, as do all C-N bonds. Ths means the positive charge has been delocalised across the ion.
[P(CH3)4]+ Analysis
Optimisation
An optimisation was run on [P(CH3)4]+ with the basis set 6-31+G(d,p). It gave bond lengths of 1.09Å (C-H) and 1.82Å (C-P). It also gave bond angles of 110.0° (P-C-H), 109.0° (H-C-H) and 109.5° (C-P-C). These, like that of the nitrogen ion correspond to a fully tetrahedral structure. The optimisation fully converged (see below). File:-P(CH3)4- OPT LAL.log. DOI:10042/21819
Item Value Threshold Converged?
Maximum Force 0.000160 0.000450 YES
RMS Force 0.000052 0.000300 YES
Maximum Displacement 0.000921 0.001800 YES
RMS Displacement 0.000292 0.001200 YES
Predicted change in Energy=-4.536339D-07
Optimization completed.
-- Stationary point found.
Frequency
The vibrational calculation performed on [P(CH3)4]+ proved that the optimisation converged to a minimum. This is shown by the three low frequencies (below) all being positive. The six low frequencies above those three are also all quite close to zero, meaing that the method used was successful.
File:-P(CH3)4- FREQ4 LAL.log. DOI:10042/21872
Low frequencies --- -21.7968 -14.0590 -0.0026 -0.0017 0.0015 40.5661 Low frequencies --- 157.9963 188.5186 190.7833
The calculation recorded 45 vibrational modes and, just like with the nitrogen ion, many of these have a very low intensity, due to there being a very small, or no change whatsoever in the dipole moment. There are 24 of these, the assumption that they are IR inactive is made. The remaining 21 vibrational modes can be split into 7 bands. Each band has 3 vibrational modes inside, but at very close frequencies. These 7 bands correspond to the 7 peaks on the IR spectrum. It is worth noting that the peaks are all more intense than that of the nitrogen ion.
![The IR spectrum of[P(CH3)4]+](/images/5/54/-P%28CH3%294-_IR_LAL.png)
There are three peaks with a very large intensity. The first of these, at 1010 cm-1, corresponds to a C-P stretch on one C-P bond, with the other three C-P bonds bending. The second large (1356 cm-1) peak correponds to an asymmetric C-P stretch, with two pairs of C-P bonds stretching out of time with each other. The final large peak (1477 cm-1) corresponds to an asymmetric C-H bend, with three methyl groups bending out of time with each other.
MOs
25 occupied orbitals were visualised for [P(CH3)4]+, this is obviously more than that of [N(CH3)4]+, which is most probably due to the extra core orbitals caused by phosphorous being in a lower period than nitrogen. The lowest orbital (P 1s) is a much lower energy as well (-77.34562 au). The first bonding orbital is energy level 9 and has an energy of -0.99485 au. The visualisation shows that it engulfs the whole molecule and is all one phase, the same as in the nitrogen ion. Interestingly, all of the hydrogen atoms are covered, whereas in the nitrogen ion the orbital does not engulf the whole of each hydrogen atom. This could be related to the fact that the hydrogens on the nitrogen ion carry a more positive charge than those on the phosphorous ion. This is discussed later in the NBO Analysis section. File:-P(CH3)4- NBO LAL.log. DOI:10042/21834
NBOs
The charge data showed charges of +0.219(H), -0.561(C) and +0.612(P). This is compared against the other data at the bottom. The range of charges shown in the thumbnail of the charge distribution is -1 to 1.
The hybridisation data shows that the C-P bond receives 41.58% of its electron density from the phosphorous atom and 58.42% from each carbon atom. This coincides with the charge density data above. The hybridisation data shows that the P atom in the C-P bonds exhibit 25% s-character and 75% p-character. This corresponds to a perfectly tetrahedral, sp3 structure.
[S(CH3)3]+ Analysis
Optimisation
The optimisation of [S(CH3)3]+ was run with the 6-31+G(d,p) basis set. The optimisation gave bond lengths of 1.82Å (C-S) and 1.09Å (C-H). It also gives bond angles of 111.0° (H-C-H), 102.8° (C-S-C) and 110.6° (H-C-S). These all correspond to a tetrahedral geometry, even though the C-S-C angle seems low, it is only this way because the sulphur lone pair takes up more space, and so pushes the substituents closer together. This optimisation converged (below)
File:-S(CH3)3- OPT LAL.log. DOI:10042/21817 .
Item Value Threshold Converged?
Maximum Force 0.000155 0.000450 YES
RMS Force 0.000045 0.000300 YES
Maximum Displacement 0.001644 0.001800 YES
RMS Displacement 0.000413 0.001200 YES
Predicted change in Energy=-2.320872D-07
Optimization completed.
-- Stationary point found.
Frequency
The frequency analysis run on the optimisation from before shows that the stationary point found is a minimum. This is proven by the presence of three positive low frequencies (below). The other six frequencies prove the method used was appropriate because they are all within ±20cm-1. File:-S(CH3)3- FREQ LAL.log. DOI:10042/21820 .
Low frequencies --- -10.0392 -1.1614 -0.0034 -0.0033 0.0010 16.1748 Low frequencies --- 160.5961 198.1711 198.5242
The vibrational analysis shows that there are 33 vibrational modes, unlike the 45 present in the previous two ions. This would be down to the absence of an extra methyl group present in the phosphorous and nitrogen ions. There are also a lot less IR inactive modes, most likely due to the fact that this ion has a dipole moment, so symmetric vibrational modes that did not create a change in dipole moment in the other two actually do here. This is shown by a more complicated IR spectrum. There are 4 major peaks in the spectrum. The first is at 623 cm-1 and corresponds to a symmetric C-S stretch. The second main peak is at 1069 cm-1 and has an intensity of 11. This peak is a combination of two asymmetric bends, the C-S groups are bending together, but out of time, in the other bend, two of the C-S bonds are bending vigorously, and the other not as much. The third main peak is a combination of three modes. The first (1468 cm-1, 26) is an asymmetric C-H bend, as is the second (1469 cm-1, 27), whereas the third (1479 cm-1, 43) is a symmetric C-H bend. The final main peak (3182 cm-1, 8) on the IR spectrum is a combination of two asymmetric C-H stretches of the same energy.
![An IR Spectrum of S(CH3)3]+.](/images/c/c5/-S%28CH3%293-_IR_LAL.png)
MO Analysis
Population analysis was run on the ion from the frequency checkpoint file, the analysis produced 21 occupied orbitals, which differed from those produced from the nitrogen ion slightly. This is mostly due to the fact that sulphur is a heavier element, and it also has a lone pair. The first non-core bonding orbital is at energy level 9, not 6. This is due to the presence of 2s, 2px, 2py and 2pz core orbitals. The presence of the lone pair means that the orbitals are much more delocalised and there are more through space interactions.
File:-S(CH3)3- MO LAL.log. DOI:10042/21835
NBO Analysis
The NBO analysis (summarised below) showed that the carbon hybrid orbital experienced different s and p character for different bonds. for one C-H bond in each methyl group the hybrid orbital had 27.66% s-character and 72.29% p-character. For the other two C-H carbon hybrids there was 26.94% and 73.01% s and p character respectively. This could be down to the effect of the lone pair. The C-S bond hybridisation was shown to be 18.44% s-character and 81.41% p-character w.r.t. carbon and 17.07% s-character and 82.32% p-character w.r.t. sulphur. It also showed that the lone pair was hybridised with 48.79% s-character and 51.20% p-character.
NBO Analysis
| NBO Charge Distribution Comparison (Range: -1 to 1) | |||
|---|---|---|---|
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
 |
 |
| |
| C | -0.485 | -0.561 | -0.845 |
| H | +0.273 | +0.219 | +0.287 |
| X | -0.341 | +0.612 | +0.851 |
The three ions all have positive charges on the hydrogen atoms, with similar magnitudes. The carbon atoms are also all negative, with similar charges for P and N and slightly lower for the S ion. The interesting part is the charges on the central atoms (X). For [N(CH3)4]+, the nitrogen is negative, whereas the othertwo X atoms are positive. This is most probable due to the fact that nitrogen is very electronegative, and so will draw a lot of the electron density towards it. This is ratified by having slightly more positive hydrogen atoms. This also explains the lower charge on the carbon atoms in the phosphorous compound, which is a much less electronegative element. In addition, carbon is more electronegative than phosphorous, meaning the presence of 4 C-P bonds will draw a lot of electron density from the P atom, explaining the fact it has a positive charge. Using this logic however we would assume that sulphur would have a lower charge than phosphorous. This is not the case, it is much more positive and its bonded carbon atoms are much more negative. This is most likely due to sigma-conjugation with the lone pair on the sulphur. The lone pair density is donated into the σ* C-H orbital. This reduces the amount of electron density on the sulphur, and increases the electron density on the carbon atoms.
| NBO C-X Contribution Comparison | |||
|---|---|---|---|
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
| C | 33.21% | 58.42% | 47.66% |
| X | 66.79% | 41.58% | 52.34% |
The contribution of electron density supports the data above. The electron density in the C-N bond is dominated by the nitrogen atom, which corresponds to the negative charge shown in the charge distribution diagram. The carbon atom contributes more electron density in the C-P bonds, meaning there is more electron density situated on them, considering that there are four carbon atoms bonded to the phosphorous, this explains its positive charge. With regards to the C-S bond, the lone pair sigma conjugation the sulphur will add to its contribution percentage and so will skew the contribution percentage towards the sulphur atom.
The traditional description of an alkyl nitronium ion shows the charge located solely on the nitrogen atom. By using the data for [N(CH3)4]+, we can see that the positive charge is actually shared between the hydrogen atoms. This is demonstrated by the green colour in the charge distribution diagram. The hydrogen atoms were also shown to have a charge of +0.273. Multiplying this by 12 and subtracting the negative charges on the nitrogen and carbon atoms give a charge of 1. Because the hydrogen atoms all have the same charge, the charge is delocalised across the whole ion.
[N(CH3)3(CHOH)]+
Optimisation
An optimisation was run on [N(CH3)3(CHOH)]+ using the 6-31+G(d,p) basis set. The calculation was not run on the HPC due to a large queue. The optimisation gave bond lengths of 1.51Å (C-N), 1.09Å (C-H), 1.39Å (C-O) and 0.97Å (O-H). It also gave bond angles of 109.8° (H-C-H), 108.5° (H-C-N), 108.3° (C-N-C), 106.3° (N-C-O) and 111.2° (C-O-H). The oxygen atom has 2 lone pairs which affect the bond angles with which it is involved. As can be seen below, the optimisation converged. File:-N(CH3)3(CHOH)-+ OPT2 LAL.LOG.
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.001449 0.001800 YES
RMS Displacement 0.000444 0.001200 YES
Predicted change in Energy=-3.373257D-08
Optimization completed.
-- Stationary point found.
Frequency
A frequency calculation was carried out on the ion. The operation was not carried out on the HPC due to a large queue. The excerpt from the log file (below) shows that the optimisation converged on a minimum and also that the method used was good enough. File:-N(CH3)3(CHOH)-+ FREQ LAL.LOG.
Low frequencies --- -32.7062 -22.5582 -10.5568 -0.0001 0.0005 0.0008 Low frequencies --- 3.1601 118.7628 207.5952
The frequency analysis recorded 48 vibrational modes, most of which were IR active. This will be because the ion is less symmetrical. The IR spectrum is also much more complicated. There are a few large peaks, such as one at 885 cm-1 (I=67). The main movement in this mode is the C-N stretch. The next large peak is at 1113 cm-1 (I=52). This corresponds to a C-O stretch. The polar nature of these bonds means that when they are disturbed they causes a large change in dipole moment, giving a high intensity. The next large intensity peak is situated at 1203 cm-1 (I=56) and indicates an O-H bend. There are 4 small intensity peaks around 3090 cm-1 that correspond to symmetrical C-H stretches closely followed by an asymmetrical C-H stretch. The final, largest peak corresponds to an O-H stretch, this obviously has the largest change in dipole moment (3827 cm-1 I=156).
There was also another vibrational mode recorded, at a frequency of -32 cm-1. The mode corresponded to a bending of the O-H bond. When gaussian suggests a negative vibration, it is usually a sign that increasing the displacement of this vibration will lower the energy of the compound. This vibration could possibly suggest that the dissociation of the hydrogen could lower the overall energy of the compound by neutralising the charge. This proton could potentially dissociate to form a zwitterion.
On the other hand there could be an issue with the B3LYP method, and potentially using a simpler method, such as hartree-fock could eliminate this vibration.
MOs
File:-N(CH3)3(CHOH)-+ FREQ MOIN.LOG. DOI:10042/22028
NBOs
The NBO analysis shows that the addition of an alcohol group to one of the methyl groups causes a major change to the charge distribution. at first we see that the carbon attached to the oxygen becomes positive, rather than negative like the others. It also removes the uniformity of the charge, and almost every atom now has a unique charge. The charge distribution was visualised with a range of -0.8 to 0.8.
The NBO data shows that the oxygen atom donates 65.67% of the electron density to the C-O bond (C=34.33%). The oxygen donates 77.42% to the O-H bond as well. Interestingly, the two lone pairs on the oxygen atom have different hybridisations. One has 47.67% s-character and 52.26% p-character (indication sp hybridisation) and the other has 99.90% p-character, which we can assume is in a p orbital. this is particularly interesting considering the hybridisation of oxygen in the C-O bond is 30.10% s-character and 69.81% p-character. This indicates sp2 hybridisation. Finally the oxygen in the O-H bond appears to show sp3 hybridisation with 22.24% s-character and 77.69% p-character.
[N(CH3)3(CHCN)]+
Optimisation
The optimisation for [N(CH3)3(CHCN)]+ was run with the 6-31+G(d,p) basis set. It gave bond lengths of 1.16Å (C≡N), 1.46Å (C-C), 1.53Å (C-N) and 1.09Å (C-H). It also reported bond angles of 179.5° (N-C-C), 110.2° (C-C-H), 107.8° (H-C-N), 107.8° (trans- C-N-C), 110.2° (cis- C-N-C), 108.8° (H-C-H: on CH2 group) and 110.2° (H-C-H).
The opimisation converged, as shown below. File:(CHCN)+ OPT2 LAL.log. DOI:10042/21938 .
Item Value Threshold Converged?
Maximum Force 0.000020 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.001228 0.001800 YES
RMS Displacement 0.000280 0.001200 YES
Predicted change in Energy=-1.120056D-08
Optimization completed.
-- Stationary point found.
Frequencies
The frequency calculation shows that the optimisation has found a minimum, and also that the method used was good. File:(CHCN)+ FREQ LAL.log. DOI:10042/21945 .
Low frequencies --- -11.7719 -1.4080 0.0008 0.0010 0.0012 12.9607 Low frequencies --- 88.4608 152.2690 206.6018
The vibrational analysis showed that there was a very large dipole moment associated with this ion (10.12 D). This is due to the electron withdrawing group (CN).
| [N(CH3)3(CHCN)]+ Vibration Spectrum | |||
|---|---|---|---|
| Mode # | Vibration Form | Frequency (cm-1) | Intensity |
| 1 | Twist in the H2C-N bond | 88 | 6 |
| 2 | C≡N bend. | 152 | 8 |
| 14 | -H2C-N- stretch | 895 | 28 |
| 15 | Asymmetric H3C-N-CH3 stretches | 910 | 19 |
| 17 | trans-H3C-N- stretch | 988 | 21 |
| 36 | All C-H bonds bending asymmetrically | 1529 | 61 |
| 37 | C≡N Stretch | 2377 | 6 |
| 42 | Asymmetric C-H stretch | 3144 | 3 |
The main vibrational peaks are summarised in the above table. The most intense peaks are that with a lot of functional groups involved. This would explain the low intensity of the C≡N stretch.
MO
There are 27 occupied molecular orbitals in [N(CH3)3(CHCN)]+. This includes 7 core orbitals. The lowest bonding orbital bears resemblance to previous examples in that it engulfs a lot of the molecule and is one single phase, however unlike the other ions discussed previously, not the whole compound is involved; the -CN group lies outside this orbital. Similarly to the [N(CH3)4]+ ion, the hydrogen atoms also are not engulfed in electron density.
File:(CHCN)+ MOOUT LAL.log. DOI:10042/22017 .
NBO
The charge distribution analysis for [N(CH3)3(CHCN)]+ is summarised in the table below. The charge distribution was visualised in the range -0.8 to 0.8.
The hybridisation of the cyanide group is consistent with sp hybridisation, withtwo of the bonds having mostly p-character, and the N lone pair and the sigma bond having 56% s-character and 44% p-character. The hybridisation data for the other bonds all show a tetrahedral, sp3 character.
Comparisons
Charge Distribution
The charge distribution data for [N(CH3)4]+, [N(CH3)3(CHCN)]+ and [N(CH3)3(CHOH)]+ are below. From this we can see the effect on the delocalisation of the charge in N(CH3)4]+ by adding an electron withdrawing group (-CN) and an electron donating group (-OH). Firstly, the effect on the charge of the central atom is negligible in the case of the -OH substituent, however the -CN compound has a much more negative charge on the central nitrogen. Next we can see that the addition of both groups has caused the carbon with which it is bonded to change sign, with the oxygen bonded carbon actuall being more positive. This is expected though, as it is bonded directly to a highly electronegative element. The more important piece of information is the charge on the two hydrogen atoms bonded to this carbon. The electron withdrawing properties of the cyanide substituent cause the charge on these to be +0.241, as opposed to the -OH group, which actually donates electron density to these hydrogens, causing them to be +0.183. Compare this to the equilibrium hydrogen charge in [N(CH3)4]+ of +0.209 and we have a direct example of the consequences of adding an electron donating or withdrawing group. This is also shown in the data for the trans- methyl group. The carbon in this group is much more negative in the -OH compound than that in the -CN compound, indicating that they donate and withdraw electron density respectively. The cis- methyl groups don't seem to be affected as much by these effects, with both substuted compounds having similar charges for these carbons. they are both, however, more negative than the carbons in the [N(CH3)4]+ ion. Finally, there is an indication of the electronegative O and N atoms drawing electron density through space. This is shown by the cis- hydrogens closest to the functional groups being noticeably more positive than other hydrogen atoms in the same ion, and the N(CH3)4]+ ion.
HOMO and LUMO
A comparison table of the HOMO and LUMO of [N(CH3)4]+, [N(CH3)3(CHCN)]+ and [N(CH3)3(CHOH)]+ is below. From this data we can see the changes that the introduction of substituents has made. Firstly, we can see that the energy level of the HOMO and LUMO has increased, this will be due to the increased number of electrons that are present in the molecule. We can also identify that the energy of the HOMO has increased. This can be seen by observing the HOMO of [N(CH3)4]+. It contains large orbitals with lots of delocalisation, which would lower the energy, there are also less nodes present. The HOMO for the two substituted compounds are very similar to each other. They have more nodes than the original and less delocalisation. The [N(CH3)3(CHOH)]+ HOMO is the highest in energy.
The LUMOs for each compound look much more similar, with a large area delocalised, and smaller, out of phase surfaces surrounding certain areas of each molecule. The CN group on the [N(CH3)3(CHCN)]+ ion causes this ion to differ the most, as a large surface almost engulfs the entirety of the other two molecules, the CN group breaks clear and has a more stable structure. This causes it to have a much lower energy than the other two LUMOs.
The result of this is that the two substituted compounds have a similar HOMO-LUMO gap, whereas the original [N(CH3)4]+ ion having a much larger energy gap. Chemically, this would make the [N(CH3)4]+ more stable, as the energy required to donate into the LUMO and destabilise the molecule is much greater. This is most likely caused by the extended delocalisation of this ion,which causes a uniform charge distribution across the ion, rather than uneven areas of high an low charge noticed in the other two. As an ionic liquid this could have many implications. As a solvent it would mean that the [N(CH3)4]+ ion would be best, due to its higher stability, making it more useful for use in experiments with high temperatures or high potentials.
- ↑ M. Atanasov, D. Reinen, J. Phys.Chem. A., 2001, 105, 5450-5467
- ↑ G. Frenking, S. Fau, C. Marchand, H. Grutzmacher, J. Am.Chem. Soc., 1997, 119, 6648-6655
- ↑ P. Schwerdtfeger, G. Heath, M. Dolg, M. Bennett, J. Am.Chem. Soc., 1992, 114, 7518-7527
- ↑ H. Niedermeyer, J.P. Hallet, I. J. Villar-Garcia, P. A. Hunt, T. Welton, Chem. Soc. Rev., 2012, 41, 7780-7802










![The IR spectrum of [N(CH3)3(CHOH)]+](/images/f/fd/-N%28CH3%293%28CHOH%29-%2B_IR_LAL.png)


![The IR spectrum of [N(CH3)3(CHCN)]+](/images/8/8c/-N%28CH3%293%28CHCN%29-%2B_IR_LAL.png)