Rep:Mod:lkb110mod3
Module 3: Transition States
In this exercise, gaussian shall be used to calculate transition states, activation energies and conformations to enable comparisons to made between possible reaction paths.
The Cope Rearrangement of 1,5-hexadiene
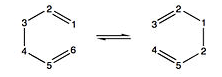

The Cope Rearrangement, shown to the right [1] , is classified as an intramolecular 3,3 sigmatropic rearrangement which can adopt either the "chair" or "boat" conformation. Activated by heat and corresponding to a 4n+2 Π system, the reaction occurs through a concerted mechanism via a Huckel transition state.
1,5-hexadiene can adopt ten different low energy conformations both in the gauche and anti-periplanar form. In the gauche conformation the largest groups, in the case the double bonds are arranged at 60 degrees to one another when viewed along the C3-C4 bond. Whereas in anti-periplanar conformers the largest groups are situated at 180 degrees from on another when viewed in the same way. This can be seen more clearly from the newman projections to the right of this page.
To assertain which are of the lowest energy, the structures were drawn on GaussView 5.0 and optimised using the "Hartree-Fock" method and the 3-21G basis set.
The calculation output is tabulated below:
| Conformer | Structure | Point Group | Energy (HF/3-21G) | Relative Energy (kcal/mol) |
Calculation output .log file |
|---|---|---|---|---|---|
| Gauche1 |  |
C2 | -231.68771613 a.u. | 3.10 | Media:GAUCHE1_OPT_321G_MON.LOG |
| Gauche2 |  |
C2 | -231.69166701 a.u. | 0.62 | Media:GAUCHE_OPT1_321G.LOG |
| Gauche3 |  |
C1 | -231.69266120 a.u. | 0 | Media:GAUCHE1_OPT_321G.LOG |
| Gauche4 |  |
C2 | -231.69153036 a.u. | 0.71 | Media:REACT_GAUCHE_OPT_321G.LOG |
| Gauche5 |  |
C1 | -231.68961573 | 1.91 | Media:GAUCHE5_OPT_321G_MON.LOG |
| Gauche6 |  |
C1 | -231.68916016 a.u. | 2.20 | Media:GAUCHE6_OPT_321G_MON.LOG |
| Anti1 |  |
C2 | -231.69260236 a.u. | 0.04 | Media:ANTI_OPT2_321G.LOG |
| Anti2 |  |
Ci | -231.69253530 a.u. | 0.08 | Media:REACT ANTI OPT 321G.LOG |
| Anti3 |  |
C2h | -231.68907066 a.u. | 2.25 | Media:ANTI3_OPT_321G_MON.LOG |
| Anti4 |  |
C1 | -231.69097054 a.u. | 1.06 | Media:ANTI_OPT4_321G.LOG |
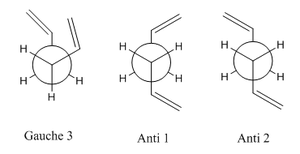
The relative energies were calculated and it can therefore be deduced that Gauche3,Anti1 and Anti2 are the lowest energy conformers of 1,5-hexadiene. From the table it can be seen that conformers Anti1 and Anti2 are slightly more destabilised than Gauche3 in the order gauche3>anti1>anti2 where gauche3 is the most stable. The gauche conformation is generally assumed to be the least stable due to the proximity of the larger substituents in the molecule and increased posibility of steric repulsion. However, in this case, gauche3 is the most stable. In this calculation a low basis set has been used which may explain these unexpected results. Therefore Gauche3, Anti1 and Anti2 will be further optimised using a higher level of theory, DFT/B3LYP/6-31G*, to give a more accurate overview of the structures.
Optimisation and Frequency Analysis of Gauche3 conformer 1,5-hexadiene using the DFT/B3LYP/6-31G* level of theory
Using the optimised structure of gauche3 1,5-hexadiene calculated earlier, the structure was further optimised using a higher level of theory and frequency analysis was done. The calculation was run through gaussian and the output linked here: Media:GAUCHE3_OPT_FREQ+631G.LOG.
A summary of the calculation is tabulated below:
| File Name | GAUCHE3_opt_freq+631g |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -234.57046507 a.u. |
| RMS Gradient Norm | 0.00000600 a.u. |
| Dipole Moment | 0.4830 Debye |
| Point Group | C1 |
| Calculation Time | 7 minute 4.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000868 0.001800 YES
RMS Displacement 0.000205 0.001200 YES
Predicted change in Energy=-3.742255D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -10.2189 -6.4944 -0.0008 -0.0004 0.0008 7.6458 Low frequencies --- 71.3146 97.7578 120.8349
These extracts show that convergence has been achieved and a minimum met. The molecule has been successfully optimised.
Optimisation and Frequency Analysis of Anti1 1,5-hexadiene conformer using DFT/B3LYP/6-31G* level of theory
Using the optimised structure of Anti1 1,5-hexadiene calculated earlier, the structure was further optimised using a higher level of theory and frequency analysis was done. The calculation was run through gaussian and the output linked here: Media:ANTI1_OPT_FREQ_631G.LOG.
A summary of the calculation is tabulated below:
| File Name | anti_opt_freq_631g |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -234.57119837 a.u. |
| RMS Gradient Norm | 0.00003290 a.u. |
| Dipole Moment | 0.2902 Debye |
| Point Group | C2 |
| Calculation Time | 6 minute 49.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000046 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000527 0.001800 YES
RMS Displacement 0.000175 0.001200 YES
Predicted change in Energy=-5.051719D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -20.8055 -8.8434 -0.0011 -0.0007 -0.0007 10.0109 Low frequencies --- 72.3096 100.1289 107.3776
These extracts show that convergence has been achieved and a minimum met. The molecule has been successfully optimised.
Optimisation and Frequency Analysis of Anti2 1,5-hexadiene conformer using DFT/B3LYP/6-31G* level of theory
Using the previously optimised Anti2 conformer, the molecule was further optimised using a higher level of theory and frequency analysis was conducted using job type "opt+freq", method "DFT/B3LYP" and basis set 6-31G*. The calculation was run through gaussian and the output linked here: Media: REACT_ANTI_OPT_FREQ_621G.LOG
A summary of the calculation is tabulated below:
| File Name | REACT_GAUCHE_OPT_FREQ_621G |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -234.57111273 a.u. |
| RMS Gradient Norm | 0.00000118 a.u. |
| Dipole Moment | 0.000 Debye |
| Point Group | Ci |
| Calculation Time | 10 minute 34.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000002 0.000015 YES
RMS Force 0.000001 0.000010 YES
Maximum Displacement 0.000053 0.000060 YES
RMS Displacement 0.000020 0.000040 YES
Predicted change in Energy=-9.670423D-11
Optimization completed.
-- Stationary point found.
Low frequencies --- -7.2270 -2.6795 -0.0010 -0.0008 -0.0007 1.8403 Low frequencies --- 71.6151 78.8116 116.4168
These extracts show that convergence has been achieved and a minimum met. The molecule has been successfully optimised.
Comparison of Low Energy Structures using a higher level of theory
Having optimised the three lowest energy conformers of 1,5-hexadiene using the DFT/B3LYP/6-31G* method, the relative energies were calculated.
| Conformer | Total Energy | Relative Energy (kcal/mol) | Experimental Relative Energy (kcal/mol) [2] |
|---|---|---|---|
| Gauche3 | -234.57046507 a.u. | 0 | 0.34 |
| Anti1 | -234.57119837 a.u. | 0.46 | 0.05 |
| Anti2 | -234.57111273 a.u. | 0.41 | 0.00 |
The above table shows that, according to experimental data, Anti1 is the lowest energy conformer. However, according to the calculations run through gaussian, gauche3 remains the lowest energy confromation. This suggests an error in the calculation of the gauche3 structure as the difference between the relative energies of anti1 and anti2 correspond to the 0.05kcal/mol difference seen from the experimental relative energies. Despite numerous attempts, I have not been able to rectify the problem with the gauche3 optimisation within the time given.
| Bond Length | Hartree Fock/3-21G Gauche3 |
DFT/B3LYP/6-31G* Gauche3 |
Hartree Fock/3-21G Anti1 |
DFT/B3LYP/6-31G* Anti1 |
Hartree Fock/3-21G Anti2 |
DFT/B3LYP/6-31G* Anti2 |
|---|---|---|---|---|---|---|
| C2-C3/C4-C5 (Å) | 1.50847 | 1.50860 | 1.50884 | 1.50769 | 1.50889 | 1.50778 |
| C3-C4 (Å) | 1.55323 | 1.55687 | 1.55237 | 1.55434 | 1.55291 | 1.55499 |
| C=C(Å) | 1.31631 | 1.34147 | 1.31610 | 1.34147 | 1.31615 | 1.34145 |
| Dihedral Angle(degrees) | 67.969 | 66.973 | 176.912 | 175.818 | 180 | 180 |
When comparing the geometries of the structures (tabulated above) the higher level calculation has not made any drastic alterations to the structure of the molecule. However these small changes in bond length and dihedral angle will ultimately change to the total energy of the molecule. No imaginary frequencies were observed for any of the optimisations showing them to be successful
Thermochemistry of 1,5-hexadiene with DFT/B3LYP/6-31G* level of theory
| Conformer | Sum of Electronic and Zero Point Energy (Hartree) | Sum of electronic and thermal energy(Hartree) | Sum of electronic and thermal enthalpy(Hartree) | Sum of electronic and thermal free energy(Hartree) |
|---|---|---|---|---|
| Anti1 | -234.428156 | -234.420873 | -234.419929 | -234.459746 |
| Anti2 | -234.428074 | -234.420768 | -234.419824 | -234.459702 |
| Gauche3 | -234.427302 | -234.420105 | -234.419161 | -234.458800 |
The optimisation of these structures at the DFT/B3LYP/6-31G* level also calculated the Energies displayed in the table above.
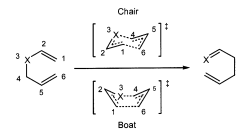
"Chair" Transition State
The Cope Rearrangement reaction can run via two possible transition states; the "chair" and the "boat" , shown in the figure to the left. Gaussian enables the calculation of the transition state structures.

Optimisation of the allyl structure
An allyl fragment was drawn on gaussian and optimised using the "Hartree-Fock" method and "3-21G" basis set. The output of the calculation is linked here: Media:ALLYL_OPT_HF_321G.LOG
The optimised is shown to the right of this page and a summary of the calculation is tabulated below.
A summary of the calculation is tabulated below:
| File Name | ALLYL_OPT_HF_321G |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | UHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Doublet |
| Total Energy | -115.82303991 a.u. |
| RMS Gradient Norm | 0.00009674 a.u. |
| Dipole Moment | 0.0293 Debye |
| Point Group | C1 |
| Calculation Time | 14.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000160 0.000450 YES
RMS Force 0.000056 0.000300 YES
Maximum Displacement 0.000711 0.001800 YES
RMS Displacement 0.000290 0.001200 YES
Predicted change in Energy=-1.860815D-07
Optimization completed.
-- Stationary point found.
Convergence has been achieved.
Optimisation of Guess "Chair" Transition State using the Hartree Fock/3-21G level of theory
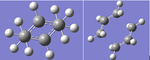
Two optimised allyl structures from the previous calculation were superimposed, with the terminal carbons spaced ~2.2 Å apart, to guess the "chair" transition state structure. An optimisation calculation was then run on the structure using method "opt +freq", optimising to a transition state (Berny). Additional keywords used were "Opt=NoEigen". The ouput of the calculation is linked here: Media:CHAIR_TS_GUESS.LOG.
A summary of the calculation is tabulated below:
| File Name | CHAIR_TS_GUESS |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.61932243 a.u. |
| RMS Gradient Norm | 0.00002546 a.u. |
| Dipole Moment | 0.0008 Debye |
| Point Group | C1 |
| Calculation Time | 14.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000035 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000845 0.001800 YES
RMS Displacement 0.000103 0.001200 YES
Predicted change in Energy=-4.366750D-08
Optimization completed.
-- Stationary point found.

Frequency analysis shows the presence of an imaginary vibration, shown below:
Low frequencies --- -818.0217 -0.0004 -0.0001 0.0007 2.9124 2.9923 Low frequencies --- 5.0173 209.6099 395.9575
The imaginary frequency at -818.0217 cm^-1 represents the maxima has been obtained on the potential energy curve and thus the transition state.
Further Optimisation of "Chair" Transition State using the Frozen coordinate method
The "Chair" transition state was further optimised using the frozen coordinate methods, where the distances between the terminal carbon atoms of the 2 allyl groups were frozen at 2.2 Å during the optimisation using the "Redundant Coord Editor". Calculation methods identical to those used for the optimisation of the "guess" chair TS were utilised for this for this calculation. i.e. the structure was optimised to "TS (berny)".
The output of this calculation is linked here: Media:CHAIR_TS_FREEZE_COORD_3.LOG
A summary of the calculation is tabulated below.
| File Name | CHAIR_TS_FREEZE_COORD_3 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.61932243 a.u. |
| RMS Gradient Norm | 0.00002549 a.u. |
| Dipole Moment | 0.0008 Debye |
| Point Group | C1 |
| Calculation Time | 6.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000072 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000420 0.001800 YES
RMS Displacement 0.000073 0.001200 YES
Predicted change in Energy=-3.637521D-08
Optimization completed.
-- Stationary point found.
Final Optimisation of "Chair" Transition State using the normal guess hessian
The removal of the frozen coordinates allows the distance itself to be optimised during this final calculation by introducing normal guess hessian. This is achieved by selecting "derivative" in the Redundant Coordinate Editor instead of "Frozen coordinate".
The output of this calculation is linked here: Media:CHAIR_TS_DERIVATIVE_MONDAY.LOG

A summary of the calculation is tabulated below.
| File Name | CHAIR_TS_DERIVATIVE_MONDAY |
| File Type | .log |
| Calculation Type | FTS |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.61932238 a.u. |
| RMS Gradient Norm | 0.00007028 a.u. |
| Dipole Moment | 0.0010 Debye |
| Point Group | C2h |
| Calculation Time | 8.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000073 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000464 0.001800 YES
RMS Displacement 0.000082 0.001200 YES
Predicted change in Energy=-2.165121D-08
Optimization completed.
-- Stationary point found.
The geometries of the optimised chair transition state are tabulated below.
| Terminal C- Terminal C | 2.01941 Å |
| Internal C-C (on allyl) | 1.38939 Å |
| Internal C-C-C angle | 120.496 degrees |
Intrinsic Reaction Coordinate
This method allows the product structure to be found by following the minimum energy pathway down to the minimum on the Potential energy surface. This is achieved by taking the optimised transition state and running a calculation on it. The job type used was "IRC", parameters were set to compute the forward reaction only (due to the symmetric nature of the reaction), to calculated the force constant "always" at each point along the IRC and to compute 50 points along the IRC. The calculation was run through gaussian and the output was linked here: Media:CHAIR_TS_IRC_2.LOG

A summary of the calculation is tabulated below.
| File Name | CHAIR_TS_IRC_2 |
| File Type | .log |
| Calculation Type | IRC |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.69157975 a.u. |
| RMS Gradient Norm | 0.00015222 a.u. |
| Dipole Moment | 0.3632 Debye |
| Point Group | C1 |
| Calculation Time | 5 minutes 36.0 seconds |
Below are the graphs presenting the IRC calculation path:
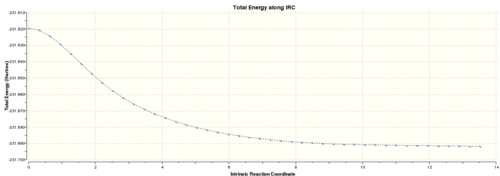
This shows the IRC to be approaching a minimum, however the RMS gradient is still relatively large suggesting it has yet to be reached. Therefore, the final structure from the IRC calculation will be minimised to reach the true minimum. Each point on the graph corresponds to the movement in the animation below.
An "opt+freq" calculation was conducted using the "Hartree-Fock" method and the "3-21G" basis set. The calculation was run through gaussian and the output linked here: Media:CHAIR_IRC_MINIMISE.LOG.
A summary of the calculation is tabulated below:
| File Name | Chair_IRC_minimise |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.69166702 a.u. |
| RMS Gradient Norm | 0.00000804 a.u. |
| Dipole Moment | 0.3804 Debye |
| Point Group | C2 |
| Calculation Time | 10.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.001402 0.001800 YES
RMS Displacement 0.000452 0.001200 YES
Predicted change in Energy=-7.532587D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -2.0842 -1.5920 -0.0008 -0.0008 -0.0008 0.9690 Low frequencies --- 63.6702 98.1812 113.3898
By comparison of the structure and its total energy to the conformers of 1,5-hexadiene in the table a t the top of this wikipage. It can be deduced that the product of the cope rearrangement passing through the "chair" transition state is Gauche2.
Optimisation of "Chair" Transition State using the DFT/B3LYP/6-31G* method
The starting molecule for this calculation was the optimisated chair transition state using the "Hartree-Fock" method and "3-21G" basis set. The calculation was set up using "opt+freq" job type, optimising to "TS (Berny)" with the force constant being calculated once. The method used was DFT/B3LYP/6-31G*. The calculation was run through gaussian and the output linked here: Media:CHAIR_TS_OPT_FREQ_631G.LOG

A summary of the calculation is tabulated below:
| File Name | CHAIR_TS_opt_freq_631G |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -234.51595692 a.u. |
| RMS Gradient Norm | 0.00001471 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2h |
| Calculation Time | 4 minutes 15.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000837 0.001800 YES
RMS Displacement 0.000214 0.001200 YES
Predicted change in Energy=-5.243525D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -555.7364 -0.0008 -0.0007 -0.0004 18.3528 20.8306 Low frequencies --- 36.3822 189.6868 240.2280
Again, the presence of a negative, imaginary frequency indicates the transition state has been reached.
The geometries of the optimised chair transition state are tabulated below.
| Terminal C- Terminal C | 2.05553 Å |
| Internal C-C (on allyl) | 1.40796 Å |
| Internal C-C-C angle | 121.251 degrees |
"Boat" Transition State
Optimisation of the "Boat" Transition State
A different method is used to calculate the transition state of the "boat" conformation. In this case the "QST2" method will be used. The optimised Anti2 conformation of 1,5-hexadiene with Ci symmetry(optimised in an earlier section) was taken and the numbered so that the movement of the atoms could be monitored before and after the 3,3 sigmatropic rearrangement.

The calculation was run through gaussian using Job type "opt + freq", optimising to "TS (QST2)", the method used was "Hartree-Fock" and Basis set "3-21G".
However, this calculation failed due to the vast difference between the reactant/product structures and the transition state and gaussian not recognising the need for rotation around the C-C bond.
Therefore, the by altering the dihedral angles (C2-C3-C4-C5) on the reactant/product structures to 0 degrees and the angles C2-C3-C4/C3-C4-C5 to 100 degrees and re-running the calculation (making sure the numbering exactly matches that of the diagram below, otherwise the calculation is unsuccessful).
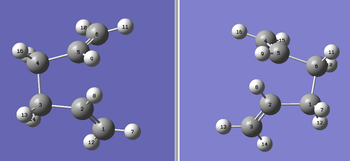
The calculation is successful and the output is linked here: Media:OPT_BOAT_QST2_321G_2.LOG.
A summary of the calculation is tabulated below.
| File Name | OPT_BOAT_QST2_321G_2 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.60280238 a.u. |
| RMS Gradient Norm | 0.00002948 a.u. |
| Dipole Moment | 0.1583 Debye |
| Point Group | C2v |
| Calculation Time | 8.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000045 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.001318 0.001800 YES
RMS Displacement 0.000349 0.001200 YES
Predicted change in Energy=-1.056572D-07
Optimization completed.
-- Stationary point found.

Frequency analysis shows the presence of an imaginary vibration, shown below:
Low frequencies --- -840.0322 -3.2450 -1.0360 -0.0005 -0.0004 0.0002 Low frequencies --- 3.0197 155.2630 382.0773
Animation of imaginary vibration reported at frequency -840.0322 cm^-1 indicates the transition state has been reached.
Intrinsic Reaction Coordinate
As with the chair transition state, the IRC is used to calculate the product structure of the cope rearrangement. This is achieved by taking the optimised transition state and running a calculation on it. The job type used was "IRC", parameters were set to compute the forward reaction only (due to the symmetric nature of the reaction), to calculated the force constant "always" at each point along the IRC and to compute 50 points along the IRC. The calculation was run through gaussian and the output was linked here: Media:BOAT_IRC.LOG

A summary of the calculation is tabulated below.
| File Name | BOAT_IRC |
| File Type | .log |
| Calculation Type | IRC |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.60280238 a.u. |
| RMS Gradient Norm | 0.00002946 a.u. |
| Dipole Moment | 1583 Debye |
| Point Group | C1 |
| Calculation Time | 6 minutes 48.0 seconds |
Below are the graphs presenting the IRC calculation path:

This shows the IRC to be approaching a minimum, however the RMS gradient is still relatively large suggesting it has yet to be reached. The below animation corresponds to the IRC graph.

An "opt+freq" calculation was conducted using the "Hartree-Fock" method and the "3-21G" basis set to reach the true minimum. The calculation was run through gaussian and the output linked here: Media:CHAIR_IRC_MINIMISE.LOG.
A summary of the calculation is tabulated below:
| File Name | boat_irc_minimise |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -231.69266120 a.u. |
| RMS Gradient Norm | 0.00000496 a.u. |
| Dipole Moment | 0.3406 Debye |
| Point Group | C1 |
| Calculation Time | 9.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.001762 0.001800 YES
RMS Displacement 0.000466 0.001200 YES
Predicted change in Energy=-1.371128D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -1.9359 -0.0144 -0.0005 -0.0003 0.0005 2.0646 Low frequencies --- 74.5844 104.9906 130.5564
By comparison of the structure and its total energy to the conformers of 1,5-hexadiene in the table a t the top of this wikipage. It can be deduced that the product of the cope rearrangement passing through the "chair" transition state is Gauche3.
Optimisation of "Boat" Transition State using the DFT/B3LYP/6-31G* method
The starting molecule for this calculation was the optimisated boat transition state using the "Hartree-Fock" method and "3-21G" basis set. The calculation was set up using "opt+freq" job type, optimising to "TS (Berny)" with the force constant being calculated once. The method used was DFT/B3LYP/6-31G*. The calculation was run through gaussian and the output linked here: Media:BOAT_TS_OPT_631G.LOG
A summary of the calculation is tabulated below:
| File Name | boat_ts_opt_631G |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -234.50425982 a.u. |
| RMS Gradient Norm | 0.00000807 a.u. |
| Dipole Moment | 0.0123 Debye |
| Point Group | C2v |
| Calculation Time | 4 minutes 15.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000405 0.001800 YES
RMS Displacement 0.000104 0.001200 YES
Predicted change in Energy=-1.682190D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -475.9916 -16.5061 -0.0003 0.0003 0.0011 10.9975 Low frequencies --- 19.3829 133.3606 247.4065
Imaginary frequency has been found signifying the transition state has been reached.
The geometries of the optimised boat transition state is tabulated below.
| Terminal C- Terminal C | 2.29118 Å |
| Internal C-C (on allyl) | 1.39617 Å |
| Internal C-C-C angle | 123.158 degrees |
Activation Energy of the "boat" and "chair" transition states
The activation energies of the two reaction paths were calculated by subtracting the energy of the starting material, in this case the Anti2 conformation of 1,5-hexadiene, from the energy of the transition states.
The results are tabulated below:
| Electronic energy (HF/3-21G) | Sum of electronic and zero-point energies(HF/3-21G) | Sum of electronic and thermal energies(HF/3-21G) | Electronic energy(B3LYP/6-31G*) | Sum of electronic and zero-point energies(B3LYP/6-31G*) | Sum of electronic and thermal energies(B3LYP/6-31G*) | |
|---|---|---|---|---|---|---|
| at 0K | at 298.15K | at 0K | at 298.15K | |||
| Chair TS | -231.61932238 a.u | -231.466702 a.u. | -231.461342 a.u. | -234.51595692 a.u. | -234.373713 a.u. | -234.367725 a.u. |
| Boat TS | -231.60280238 a.u | -231.450928 a.u. | -231.445299a.u. | -234.50425982 a.u. | -234.363284 a.u. | -234.356891 a.u. |
| Reactant (Anti2) | -231.69253530 a.u | -231.539540 a.u. | -231.532566 a.u. | -234.57111273 a.u | -234.428074 a.u. | -234.420768 a.u. |
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Experimental [4] | |
|---|---|---|---|---|---|
| at 0k | at 298.15K | at 0K | at 298.15K | at 0K | |
| ΔE (Chair) (kcal/mol) | 41.33 | 44.69 | 34.11 | 33.28 | 33.5 ± 0.5 |
| ΔE (Boat) (kcal/mol) | 55.60 | 54.76 | 40.66 | 40.08 | 44.7 ± 2.0 |
From these activation energies it can be deduced that the "chair" transition state has the lowest activation energy and therefore the lowest energy route to the products.
Cis Butadiene and Ethylene: Diels Alder Cycloaddition
Optimisation of Cis Butadiene
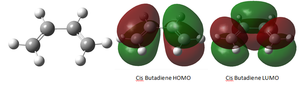
Cis-butadiene was built on gaussian and optimised using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file linked here: Media:CISBUTADIENE_OPT_SE_AM1.LOG.

A summary of the calculation is tabulated below.
| File Name | CISBUTADIENE_OPT_SE_AM1 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.04879734 a.u. |
| RMS Gradient Norm | 0.00008900 a.u. |
| Dipole Moment | 0.0414 Debye |
| Point Group | C2v |
| Calculation Time | 7.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000159 0.000450 YES
RMS Force 0.000051 0.000300 YES
Maximum Displacement 0.000768 0.001800 YES
RMS Displacement 0.000254 0.001200 YES
Predicted change in Energy=-1.540730D-07
Optimization completed.
-- Stationary point found.
Therefore it can be seen that the HOMO of cis-butadiene is asymmetric with respect to the plane whilst the LUMO is symmetric.
Optimisation of Cis-butadiene/ethylene transition state
A guess structure of the transition state between cis-butadiene and ethylene was built on gaussian, shown to the right. This structure was then optimised to a transition state using the "opt +freq" job type, the "Semi Empirical/AM1" method and key words "Opt=NoEigen. The calculation was run through gaussian and the output file linked here: Media:DIELS_ALDER_TS_OPT_GUESS.LOG

A summary of the calculation is tabulated below.
| File Name | DIELS_ALDER_TS_OPT_GUESS |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.11033724 a.u. |
| RMS Gradient Norm | 0.00000955 a.u. |
| Dipole Moment | 0.8379 Debye |
| Point Group | Cs |
| Calculation Time | 18.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000022 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000751 0.001800 YES
RMS Displacement 0.000231 0.001200 YES
Predicted change in Energy=-1.254035D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -951.8029 -13.3745 -8.7621 -5.1549 0.0024 0.0433 Low frequencies --- 0.0789 147.9895 244.8410
The transition state has been reached therefore an imaginary frequency is present at -951.8029 cm^-1, An animation of this frequency is shown below.


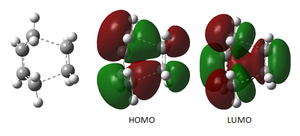
From this animation is can be deduced that the bond formation is synchronous. However when compared to the lowest positive vibrational frequency (shown to the right) this vibration displaces the position of the terminal carbon atoms hindering the bond formation.
The geometries of the transition state are tabulated below.
| Partially formed C=C | 1.39777Å |
| Partially formed C-C | 2.11807Å |
Stated here are literature values of sp3 C-C(1.330Å) and sp2 C=C (1.443Å)[5] These are longer than the calculated lengths recorded above which makes sense as the calculated bond lengths have yet to fully form. The van der waals radius of a carbon atom is 1.7Å [6]. The partially formed C-C bond is outside of this range showing the molecules need to move into position before the van der waals can have some effect.
Intrinsic Reaction Coordinate
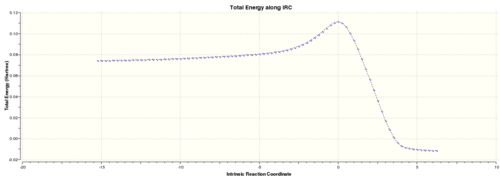
The IRC method was used to find the product structure by following the minimum energy pathway down to the minimum on the Potential energy surface. The job type used was "IRC", parameters were set to compute the reaction both ways, to calculated the force constant "always" at each point along the IRC and to compute 100 points along the IRC. The calculation was run through gaussian and the output was linked here: Media:DIELS_ALDER_IRC.LOG
A summary of the calculation is tabulated below.
| File Name | DIELS_ALDER_IRC |
| File Type | .log |
| Calculation Type | IRC |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | 0.07462541 a.u. |
| RMS Gradient Norm | 0.00005683 a.u. |
| Dipole Moment | 0.0374 Debye |
| Point Group | C1 |
| Calculation Time | 2 minutes 57.0 seconds |
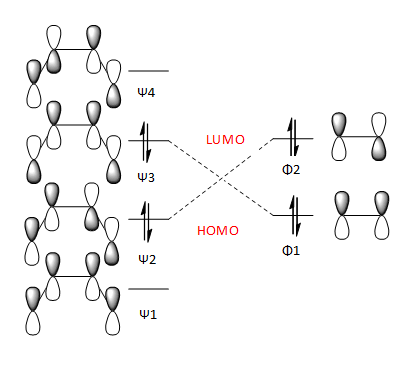
Below is the animation of the IRC calculation. It shows the ethylene approaching the cis-butadiene from above the plane of the molecule leading to maximum overlap of the appropriate orbitals shown in the FMO diagram below.

However the true minimum has yet to be reached, therefore an optimisation was conducted on the lowest energy structure of the IRC calculation using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file linked here: Media: DIELS_ALDER_IRC_MINIMISE.LOG

A summary of the calculation is tabulated below.
| File Name | DIELS_ALDER_TS_OPT_GUESS |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.11033724 a.u. |
| RMS Gradient Norm | 0.00000955 a.u. |
| Dipole Moment | 0.8379 Debye |
| Point Group | Cs |
| Calculation Time | 18.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000056 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000642 0.001800 YES
RMS Displacement 0.000164 0.001200 YES
Predicted change in Energy=-1.724417D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -52.1723 -0.0939 -0.0119 -0.0026 5.3720 5.9734 Low frequencies --- 6.1299 152.5614 381.0175
Imaginary frequency found.
Cyclohexa-1,3-diene and maleic anhydride: Regioselectivity of Diels-Alder Cycloaddition
The Diels-Alder reaction between Cyclohexa-1,3-diene and maleic anhydride has two possible routes and products, endo and exo, depending on which orientation maleic anhydride adopts when approaching Cyclohexa-1,3-diene.
Optimisation of Cyclohexa-1,3-diene using "Semi-Empirical/AM1" method
The Cyclohexa-1,3-diene molecule was built on gaussian and an optimisation calculation was run using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file was linked here: Media:CYCLOHEXADIENE_OPT_AM1.LOG

A summary of the calculation is tabulated below.
| File Name | CYCLOHEXADIENE_OPT_AM1 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | 0.02795815 a.u. |
| RMS Gradient Norm | 0.00005245 a.u. |
| Dipole Moment | 0.4559 Debye |
| Point Group | C2v |
| Calculation Time | 7.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000148 0.000450 YES
RMS Force 0.000031 0.000300 YES
Maximum Displacement 0.001095 0.001800 YES
RMS Displacement 0.000274 0.001200 YES
Predicted change in Energy=-2.131341D-07
Optimization completed.
-- Stationary point found.
Convergence was achieved and the molecule optimised.
Optimisation of maleic anhydride using "Semi-Empirical/AM1" method
The maleic anhydride molecule was built on gaussian and an optimisation calculation was run using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file was linked here: Media:MALEIC_ANHYDRIDE_SE_AM1.LOG
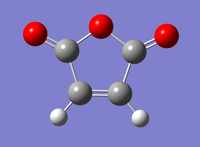
A summary of the calculation is tabulated below.
| File Name | MALEIC_ANHYDRIDE_SE_AM1 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | -0.12182305 a.u. |
| RMS Gradient Norm | 0.00018525 a.u. |
| Dipole Moment | 4.5857 Debye |
| Point Group | Cs |
| Calculation Time | 7.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000328 0.000450 YES
RMS Force 0.000143 0.000300 YES
Maximum Displacement 0.001747 0.001800 YES
RMS Displacement 0.000769 0.001200 YES
Predicted change in Energy=-9.223066D-07
Optimization completed.
-- Stationary point found.
Convergence was achieved and the molecule optimised.
Optimisation of Exo-Transition state

A guess structure of the transition state between Cyclohexa-1,3-diene and maleic anhydride was built on gaussian, shown to the right. This structure was then optimised to a (Berney) transition state using the "opt+freq" job type, the "Semi Empirical/AM1" method and key words "Opt=NoEigen. The calculation was run through gaussian and the output file linked here: Media:TS2_OPT_BERNEY_SE_AM1.LOG
A summary of the calculation is tabulated below.
| File Name | TS2_OPT_BERNEY_SE_AM1 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | -0.05041985 a.u. |
| RMS Gradient Norm | 0.00000400 a.u. |
| Dipole Moment | 5.5642 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000410 0.001800 YES
RMS Displacement 0.000081 0.001200 YES
Predicted change in Energy=-5.320134D-09
Optimization completed.
-- Stationary point found.
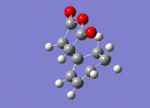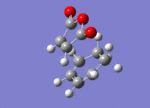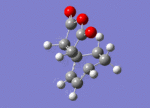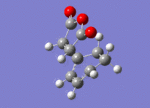
Low frequencies --- -812.2483 -1.1462 -1.0021 -0.0047 0.3167 1.3147 Low frequencies --- 2.2276 60.8490 123.8618
An imaginary frequency is present at -812.2483 cm^-1 (animation displayed to the right) indicates the transition state has been reached and the synchronous bond formation.
Intrinsic Reaction Coordinate of Exo-Transition state
The IRC method was used to find the product structure by following the minimum energy pathway down to the minimum on the Potential energy surface. The job type used was "IRC", parameters were set to compute the reaction both ways, to calculated the force constant "always" at each point along the IRC and to compute 100 points along the IRC. The calculation was run through gaussian and the output was linked here: Media:TS2_IRC_SE_AM1.LOG
A summary of the calculation is tabulated below.
| File Name | TS2_IRC_SE_AM1 |
| File Type | .log |
| Calculation Type | IRC |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -0.09537180 a.u. |
| RMS Gradient Norm | 0.00005960 a.u. |
| Dipole Moment | 4.0209 Debye |
| Point Group | C1 |
| Calculation Time | 3 minutes 43.0 seconds |
Below are the graphs presenting the IRC calculation path:
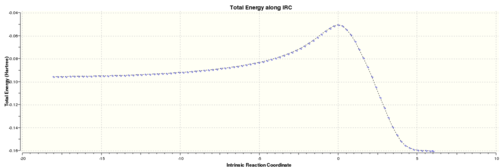
However the true minimum has yet to be reached, therefore an optimisation was conducted on the lowest energy structure of the IRC calculation using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file linked here: Media: TS2_IRC_MINIMISE.LOG
A summary of the calculation is tabulated below.
| File Name | TS2_IRC_MINIMISE |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | -0.15990937 a.u. |
| RMS Gradient Norm | 0.00001528 a.u. |
| Dipole Moment | 5.2575 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000058 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000699 0.001800 YES
RMS Displacement 0.000135 0.001200 YES
Predicted change in Energy=-4.346033D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -3.9529 -3.6263 -2.6022 -0.0027 0.0805 0.1094 Low frequencies --- 70.6002 148.5920 168.7367
Imaginary frequency found.
Optimisation of Endo-Transition state
A guess structure of the transition state between Cyclohexa-1,3-diene and maleic anhydride was built on gaussian, shown to the right. This structure was then optimised to a (Berney) transition state using the "opt+freq" job type, the "Semi Empirical/AM1" method and key words "Opt=NoEigen. The calculation was run through gaussian and the output file linked here: Media:TS1_OPT_BERNY_SE_AM1.LOG
A summary of the calculation is tabulated below.
| File Name | TS1_OPT_BERNEY_SE_AM1 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | -0.05150451 a.u. |
| RMS Gradient Norm | 0.00002717 a.u. |
| Dipole Moment | 6.1649 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000058 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.001631 0.001800 YES
RMS Displacement 0.000394 0.001200 YES
Predicted change in Energy=-9.173070D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -806.7387 -1.5281 -0.3702 -0.0104 0.3966 2.1554 Low frequencies --- 3.1366 62.4555 111.7326
Therefore an imaginary frequency is present at -806.7387 cm^-1, an animation of said frequency is shown below.
Intrinsic Reaction Coordinate of Endo-Transition state
The IRC method was used to find the product structure by following the minimum energy pathway down to the minimum on the Potential energy surface. The job type used was "IRC", parameters were set to compute the reaction both ways, to calculated the force constant "always" at each point along the IRC and to compute 100 points along the IRC. The calculation was run through gaussian and the output was linked here: Media:TS1_IRC_SE_AM1.LOG
A summary of the calculation is tabulated below.
| File Name | TS1_IRC_SE_AM1 |
| File Type | .log |
| Calculation Type | IRC |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -0.09427768 a.u. |
| RMS Gradient Norm | 0.00006661 a.u. |
| Dipole Moment | 4.8193 Debye |
| Point Group | C1 |
| Calculation Time | 3 minutes 56.0 seconds |
Below are the graphs presenting the IRC calculation path:
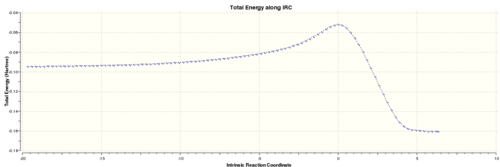
However the true minimum has yet to be reached, therefore an optimisation was conducted on the lowest energy structure of the IRC calculation using the "Semi-Empirical/AM1" method. The calculation was run through gaussian and the output file linked here: Media: TS1_IRC_MINIMISE.LOG
A summary of the calculation is tabulated below.
| File Name | TS1_IRC_MINIMISE |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Charge | 0 |
| Spin | Singlet |
| E(RAM1) | -0.16017077 a.u. |
| RMS Gradient Norm | 0.00002206 a.u. |
| Dipole Moment | 5.5836 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 seconds |
Item Value Threshold Converged?
Maximum Force 0.000075 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.000940 0.001800 YES
RMS Displacement 0.000183 0.001200 YES
Predicted change in Energy=-7.998715D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -4.9303 -4.0490 -3.3054 -0.0034 0.0475 0.1101 Low frequencies --- 72.1115 148.1370 167.3502
Imaginary frequency found.
Relative Energies of the Endo/Exo Transition States
The relative energies are tabulated below.
| Conformer | Total Energy | Relative Energy (kcal/mol) |
|---|---|---|
| Endo TS | -0.05150451 a.u. | 0.68 |
| Exo TS | -0.05041985 a.u. | 0.00 |
From the relative energies it is possible to see that the endo structure is significantly higher in energy that the exo transition state which is not expected. One would expect increased steric repulsion and strain between the axial hydrogen on the cyclo-hexadiene and the bulky oxygens on the malaiec anhydride which does not occur in the endo transition state.
Comparison of HOMO/LUMO MOs of the Exo/Endo transition states
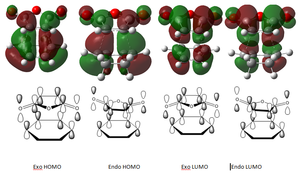
The endo conformation is favored in this reaction due to the secondary orbital overlap effect[7]. This occurs where atoms, who are not involved in the bond changing aspect of the reaction, contribute MO overlap in the HOMO transition state. This interaction occurs strongly in the endo TS but not in the exo state, thus providing further stabilization and a preference over the exo transition state. In this specific case secondary orbital overlap comes from the MOs of the (O)C-O-C(O) on the maleic anhydride which, although it is not involved in the bond making/breaking aspect of the reaction, can be seen to interact strongly in the Endo HOMO.
Conclusion
Gaussian is a very useful tool which allows the calculation and comparison of transition states, activation energies and conformations. This enables and aids understanding of many complex interactions by visually expressing them through models and animations.
References
- ↑ H. Rzepa, Pericyclic Reactions, 2nd year Imperial College London lecture course, 2012
- ↑ B. W. Gung, Z. Zhu and R. A. Fouch, J. Am. Chem. Soc., 1995, 117, 1783-1788.
- ↑ B. W.Gung,Z.Zhu,R.A.Fouch: J. Org. Chem. 2003, 68, 572-577
- ↑ M. Bearpark, https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:phys3.
- ↑ F. H. Allen, O. kennard and D. G. Watson, J. Chem. Soc. Perkin Trans., 1987, II, S7-S8.
- ↑ A.Bondi: J. Phys. Chem., 1964, 68 (3), pp 441–451
- ↑ M.A. Fox, R.Cardona, and N.J.Kiwiet: J.Org.Chem.1987,52,1469-1474