Rep:Mod:lh106module3
Module 3- The Transition State
Different configurations of 1,5-hexadiene were optimised to find which would be the most stable for the Cope Rearrangement.
Anti
| Energy, au | -231.69153035 |
| Point group, Debye | C2 |
| Same geometry/symmetry and roughly same energy as anti 1. | |
Gauche
| Energy, au | -231.69266122 |
| Point group, Debye | C1 |
| Same energy as Gauche 3 but the point group and geometry of Gauche 4 | |
The lowest energy conformer should be the conformer with the least strain. The Gauche form had a lower energy than the anti form and so I predict the lowest energy conformer will be a Gauche type conformer.The lowest conformation should be that where the the double bonds (of higher electron density) were furthest away from each other.
The Ci anti 2 conformation was created and optimised using HF method with 3-21G basis set. The Ci conformation gave a total energy of -231.692535 au which is in between the values obtained for the Anti and Gauche forms.
Ci Conformer |
The Ci structure was then optimised with the B3LYP method and 6-31G base set- a higher level of theory. This gave a slightly different looking molecule.
Chair 631-G |
As can be seen in the model, the geometry has changed between the theory sets, the Ci structure is a stretched out anti form whereas the molecule after B3LYP/6-31G treatment looks more like a ring.
Optimisation of Chair and Boat Forms
The Cope rearrangement reaction was investigate to find whether it proceeds via a cis or boat transition state.
The "guessed" transition state was created using two allyl fragments.
Transition state |
This transition state was then optimised using two different methods
Optimising To a Transition State- Chair Conformation
The transition state was optimised using two different methods. The first was to use the HF method and 3-21G base set but to optimise to a Berny transition state. This took a few attempts to work as the two parts to the transition state had to be correctly orientated but a transition state was finally optimised. This gave a negative wavelength, as expected for a transition state, at -817.987cm-1 which is very close to the -818cm-1 expected.
The other method was to freeze the distance between the atoms that will bond during the reaction. This gave the same molecule as in the first method but the bonds were limited to 2.2A as was determined when setting the distance. This method was then used again to optimise the bonds that had previously been frozen at 2.2A. This optimisation gave bond lengths of 1.55A and 4.36A between the atoms that were expected to bond.
Optimising To a Transition State- Boat Conformation
The boat conformation was optimised using a different method- the QST2 method. The reactant and product were made in Gaussian and numbered accordingly. The two molecules were altered to give the boat form. This took a few attempts for the QST2 calculation to run because the geometry of the molecules had to be correct. This gave an imaginary frequency at -154.81cm-1
Optimised Structures
Boat Structure
Ci Conformer |
Chair Structure
Ci Conformer |
The IRC for these structures was run, with Gaussian calculating the force constants at every point so that the minimum geometry would be reached. The chair IRC gave a movement between the terminal carbons, this was a small movement. The boat IRC gave a greater movement, this gave a rotation between the two halves of the molecule- the two fragments that were originally created.
Activation Energies
FreqChk was used to calculate the activation energy at 298.15K. For the chair conformation this eneryg was 83.288 KCal/mol and for the boat form:87.661 KCal/mol. It was predicted that the chair form should give a lower activation energy than the boat form, although the activation energies should be a lot lower, although this was taken at 0K and so will explain the difference.
Project: Diels Alder Reaction
Cis butadiene Cis butadiene was created in Gaussian and optimised using the AM! semi-empirical method.
Ethylene+Cis Butadiene Transition Structure

HOMO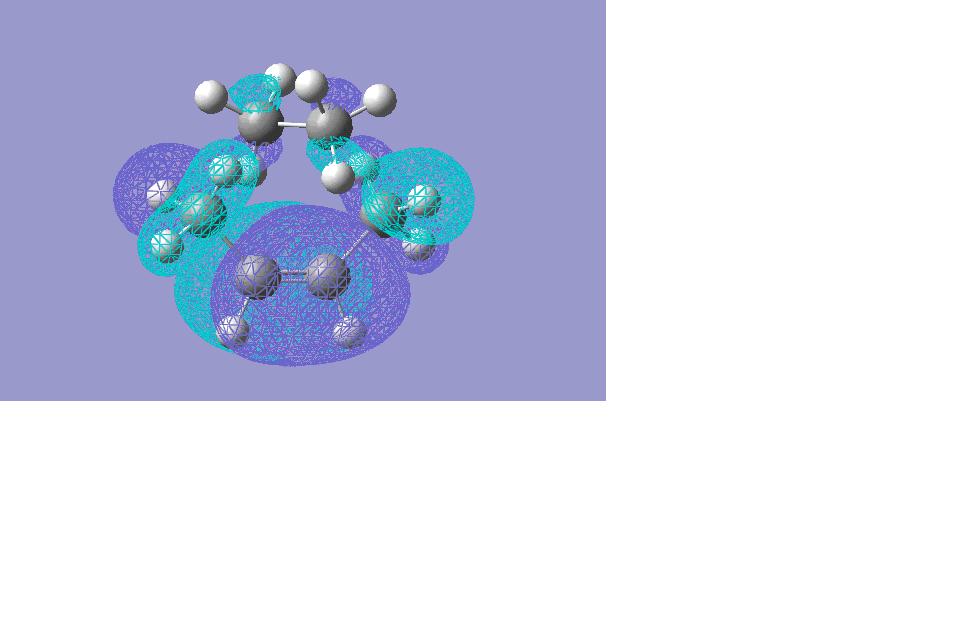
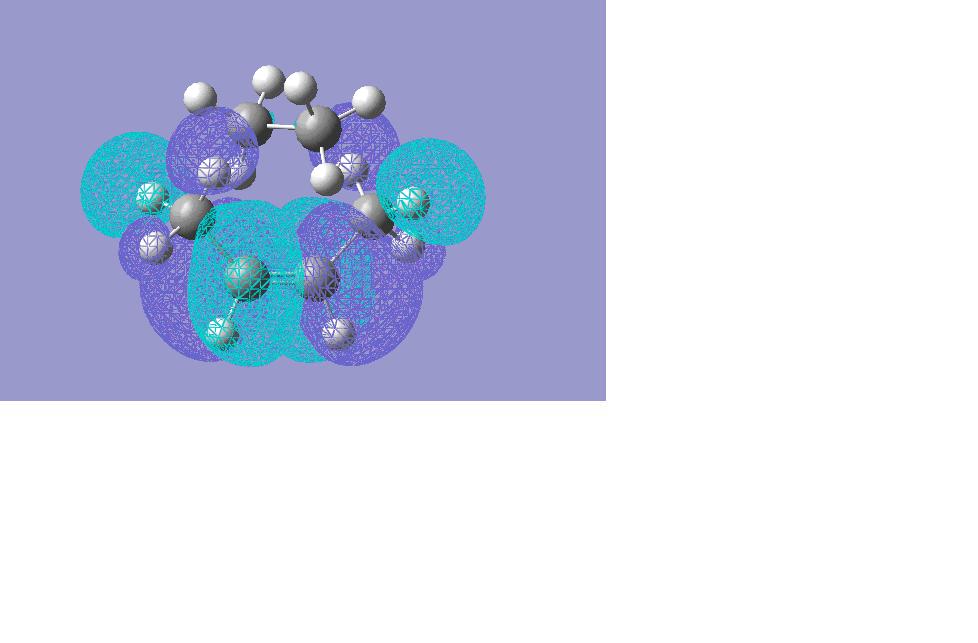
The transition frequency was found at -89.0787. The bonding is not synchronous, as vibration is only occuring at one of the bond making ends of the cis-butadiene. If both bonds were to be made at the same time, the transition state would show two vibrations- one at each end of the cis-butadiene (as two bonds are made in the final product). The orbitals used in this reaction are the HOMO and the LUMO as shown below.
The reaction between cyclohexa-1,3-diene and maleic anhydride proved more difficult to model in Gaussian. The frozen coordinate method and the transition state method (setting the calculation to calculate for a transition state) led to errors multiple times. For the exo form, the calculation (using the frozen coordinate method) ran to completion once given the following molecular orbitals but I do not believe this is the true transition state.[2]
This model did give a transition vibration frequency at -4.3 representing the two molecule s rocking from side to side. This is quite high for a transition state frequency. Also, the molecular orbitals suggest that the HOMO is carried solely by the cyclohexa-1,3-diene and the LUMO solely represents the maleic anhydride. If this was a transition state, then I would expect some interaction between the two. That is unless it is a very early transition state as that would give a molecules similar to the reactants.
The endo form gave a more satisfactory result (after many attempts). This gave 4 transition frequencies, at -223.9, -143.4, -115.2 and -25.66. These represented the vibrations as follows:
-223



For the exo form, the energy was -0.18447 whereas the endo form had an energy of -0.17979 making it slightly lower in energy and therefore more stable. This follows with the idea that the exo form is more strained because of the maleic anhydride fragment being closer to the bridging carbons which could give an interaction. This is also supported by the secondary orbital overlap as this occurs most for the endo form.
- ↑ http://books.google.co.uk/books?id=aOij0MVjsy0C&pg=PA124&dq=C-C+bond+length, Lets Review: Chemistry, the Physical Setting, Albert S Tarendash, 2001
- ↑ DOI:10042/to-1554


