Rep:Mod:lh106module2project
Mini Project- Fuels of The Future
Hydrogen is being projected as a the new fuel for the future because, unlike fossil fuels, its only product is water. Hydrogen can be used to release energy when it reacts with oxygen. Of course at room temperature hydrogen is a gas and so is difficult to store as it takes up a lot of room. One way to do this would be to increase the pressure of the hydrogen as the amount of space taken up by hydgogen gas would be lowered:. Another way would be to change the phase the hydrogen is stored in, that is to store the hydrogen as a liquid or a solid. Obviously as a liquid is going to be easier but would still require too cold a temperature for realistic use of liquid hydrogen (boiling point=-238.4°[1]. There is a way to carry the hydrogen via the use of a solid, even though the hydrogen itself is not solid. That would be to store a substance with a high hydrogen content that would be easy to get to.
One molecule that is being explored for this purpose is NH3BH3 because of the large number of hydrogens it contains.[2]. It is also meant to be stable at room temperature, and so could be easily stored. Computational methods will be used to explore how stable the molecule is.
Structure and Stability
Ammonia Borane could exist as either an eclipsed or staggered form because of rotation around the nitrogen-borane bond- in the same fashion as the isoelectric ethane. The energies of these two forms were compared to find which geometry is taken.I expect the staggered form to be lower in energy as there is less strain between hydrogens as they are further away.
| Form | Energy, au | Dipole Moment, Debye | Symmetry |
| Staggered | -83.18805550 | 5.8939 | C3V |
| Eclipsed | -83.18425903 | 5.9916 | C3V |
The staggered formation does have a lower energy, but the difference between the two is very small. The staggered formation is lower in energy because of bond-electron repulsions between the hydrogens as shown by the pink arrows below.
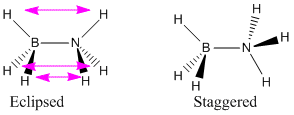
Comparison With Ethane
As previously mentioned, ammonia borane is isoelectric with ethane and so I woudl expect the two to have similar properties, and similar energies. The energy of staggered and eclipsed ethane was calculated, again using the Gaussian program with a DFT B3LYP method and 3-21G base set to give quite a rough result as it is only being used as a comparison.
| Form | Energy, au | Dipole Moment, Debye |
| Staggered | -79.80931774 au | 0 |
| Eclipsed | -79.80451884 | 0.0001 |
The NH3BH3 molecules are slightly more stable than the ethane, this suggests that the bonding between nitrogen and borane is stronger than in the organic analogue. Ammonia Borane also bonds differently to ethane. Ethane, having two carbon bonds bonding can covalently bond whereas ammonia borane, having an abnormal valence at the borane has dative bonding with the nitrogen acting as a lewis base. The N→B bond is quite low in energy- 17.58Kcalmol-1[3] but the molecule is still more stable than ethane, which has a bond energy of 80kcal
Reaction To Make NH3BH3 and To Afford Hydrogen
The stability of the different components of the reaction was calculated using Gaussian. This were then compared to see whether the reaction would take place.
| NH4Cl | -517.32723694 |
| NaCl | -622.55076536 |
| H2 | -1.17548239 |
| NaBH4 | -190.05019313 |
The overall energy of the reaction can be calculated by taking away the energy of the products from the energy of the reactants, in this case Etotal=(E(NH3BH3)+E(NaCl)+E(H2))-(E(NH4Cl)+E(NaBH4))
This gives an energy of 1.911007 x 10-3 au for the staggered form and 1.912835 x 10-3 au for the eclipsed isomer.
This would suggest that the reaction to create NH3BH3 is not favoured as it gives a positive energy. But this was a very small energy and so should still afford product. This also supports the ease of obtaining hydrogen from the molecule as the NH3BH3 would prefer to release its hydrogen.
Melting Point
Although isoelectric to ethane, ammonia-borane has a much higher melting point. It has been thought that this is done to the polar nature of ammonia-borane, this is supported by the high dipole of 5.8939 Debye and 5.9916 Debye calculated for the staggered and eclipsed ammonia-borane respectively. Ammonia-borane also has a great capacity for hydrogen bonding both between the borane and ammonia ends of the molecule [4]
It has been reported that the solid structure of ammonia-borane takes the form shown below: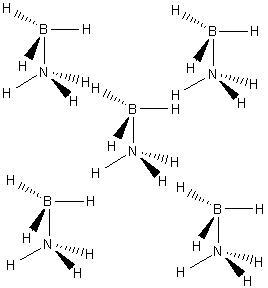
Another way to determine why ammonia-borane has a much higher melting point would be to use the CRYSTAL program in DLVisualise as this would be able to calculate the ground state energy
References
- ↑ http://www.jstor.org/sici?sici=0370-1662(1901)68%3C44%3ATBPOLH%3E2.0.CO%3B2-E&cookieSet=1 The Boiling Point of Liquid Hydrogen, Determined by Hydrogen and Helium Gas Thermometers, James Dewar, Proceedings of the Royal Society of London, Vol. 68, 1901,pg 44
- ↑ http://www.rsc.org/Publishing/ChemScience/Volume/2008/08/Borane_fuels.asp Borane leadss the way to alternative fuels, Chemical Science
- ↑ http://www.springerlink.com/content/h16940520664k3g9/fulltext.pdf G Leroy, M Sana and C Wilante, Theoretica Chimica Acta, 1993, 85, 155
- ↑ http://www.rsc.org/ej/CP/2007/b617781f.pdf Ashley C. Stowe, Wendy J. Shaw,* John C. Linehan, Benjamin Schmid and Tom Autrey, Physical Chemistry Chemical Physics, 2007, 9, 1831
