Rep:Mod:lh106module1
Hydrogenation of Cyclopentadiene Dimer
Cyclopentadiene molecules 1 and 2 were optimised to give the minimum energy using the MM2 function to give structures 1 and 2:
Structure 1 |
Structure 1b |
Structure 1 Energies
| Energy Type | Energy |
| Stretch | 1.293 |
| Bend | 20.5860 |
| Stretch-bend | -0.8413 |
| Torsion | 7.6715 |
| Non 1,4- Van der Waals | -1.4358 |
| 1,4-Van der Waals | 4.232 |
| Dipole-dipole | 0.3778 |
| Total | 31.884 |
Structure 2 Energies
| Energy Type | Energy |
| Stretch | 1.2626 |
| Bend | 20.8423 |
| Stretch-bend | -0.8406 |
| Torsion | 9.5141 |
| Non 1,4- Van der Waals | -1.5557 |
| 1,4-Van der Waals | 4.3302 |
| Dipole-dipole | 0.4490 |
| Total | 34.0018 |
The torsion energy changed the most between the two molecules, with the endo dimer having a higher torsion energy Here, the lowest energy structure, and therefore the most stable, is the 1st structure. As the second endo structure is formed then the reaction must proceed by kinetic factors- where the stability of the transition state decides which molecule is produced.
The same process was followed for structures 3 and 4
Structure 3 Energies
| Energy Type | Energy |
| Stretch | 1.6411 |
| Bend | 30.8459 |
| Stretch-bend | -1.6328 |
| Torsion | 18.9822 |
| Non 1,4- Van der Waals | -0.9547 |
| 1,4-Van der Waals | 4.8249 |
| Dipole-dipole | 0.1626 |
| Total | 53.8691 Kcal/mol |
Structure 4 Energies
| Energy Type | Energy |
| Stretch | 1.0916 |
| Bend | 14.5475 |
| Stretch-bend | -0.5461 |
| Torsion | 12.5089 |
| Non 1,4- Van der Waals | -1.0768 |
| 1,4-Van der Waals | 4.5041 |
| Dipole-dipole | 0.1406 |
| Total | 31.1699 Kcal/mol |
The lowest energy structure here was definitely the fourth structure and thus this should be the most stable form. The only energy that is greater for the fourth structure is the non-1,4 Van der Waals energy. The bending energy from structure 3 was almost double that of structure 4, torsion energy was also significantly higher for the third structure.
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring
The two pyridinium reactants were opptimised using the MM2 procedure to give the minimum energy geometries as follows:
Structure 5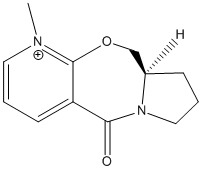 Structure 7
Structure 7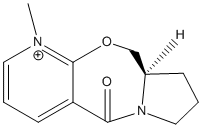
These gave quite similar energies of 27.4877 Kcal/mol for structure 5 and 26.3846 Kcal/mol for structure 7. But this energy could still be further lowered by manual manipulation of the structure; this gave an energy of 16.137 kcal/mol:
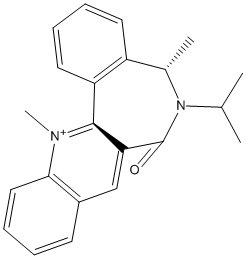
The Me group always adds as shown because it adds anti to the Hydrogen attached to the chiral centre. Also, in the case of the Grignard reagent, the MgMeI coordinates to C=O and so it is sterically easier to attach the Me group there rather in that geometry rather than facing down as the C=O faces about 45 degree up from the planar rings.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The key intermediate in the synthesis of taxol has two conformatino based around the carbonyl group; either pointing up or down. Both these confirmations were analysed using the MM2 method as shown below:
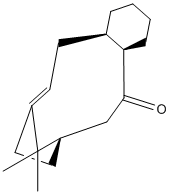
C=O pointing upwards
| Energy Type | Energy |
| Stretch | 2.8230 |
| Bend | 16.4088 |
| Stretch-bend | 0.4526 |
| Torsion | 21.4004 |
| Non 1,4- Van der Waals | -0.8536 |
| 1,4-Van der Waals | 14.0435 |
| Dipole-dipole | 0.1358 |
| Total | 54.4104 Kcal/mol |
C=O pointing downwards
| Energy Type | Energy |
| Stretch | 2.5510 |
| Bend | 10.6601 |
| Stretch-bend | 0.3221 |
| Torsion | 19.535 |
| Non 1,4- Van der Waals | -1.2750 |
| 1,4-Van der Waals | 12.5435 |
| Dipole-dipole | -0.1816 |
| Total | 44.23737 Kcal/mol |
The C=O down intermediate is the most stable as the reaction is slow, this suggests that it proceeds via thermodynamic control where the product is the more stable. Therefore structure 10 will be the key intermediate which fits with the sterics of the system- if the reaction occurs around the C=O bond it will be easier to reach if anti from the methyls from the bridging carbons.
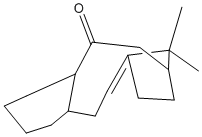
This structure was then manually manipulated to create an even more stable version of the above molecule. This only brought the total energy down by 1 kcal/mol
How One Might Induce Room Temperature Hydrolysis of A Peptide
Two isomers, 13 and 14 were analysed using MM2, two version of each isomer were observed- with the N ring subsituent in the axial and equitorial positions, 13 reacts quickly to give a peptide and 14 slowly.
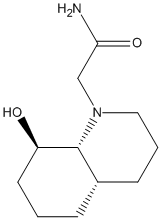
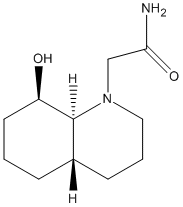
For structure 13 there was a great difference between the energies for the axial and equitorial isomers with the energy for the equitorial form being much higher. This was especially appparent in the bending energy, which was almost 4 times as high, and the torsional strain. The stretch strain was slightly higher for the axial form. From looking at the optimised structures given by the MM2 process, in the axial structure, the nitrogen is away from the decalin ring and away from the OH group, with the C=O facing the oxygen.
Regioselective Addition of Dichlorocarbene
The geometry and energies of dichlorocarbene were explored using three different methods: 1)MM2 (molecular mechanics)- this method had already been used for the examples above. This is a mechanical approximation and so can not cope with complicated molecules accurately. 2)HF method with STO-3G base set- this is more accurate and gives a molecular orbital approach to finding the valence electron molecular wavefunction. This was also used to explore the different energy levels, in particular the HOMO. 3) B3LYP method with 6-31G base set- this is the quantum mechanical treatment of the wavefunction and geometry. This method was also used to look at the vibrations of the molecule.
Molecular Mechanics Method
The total energy for the molecule obtained by the MM2 method was 17.8983 Kcal/mol.
HF/STO-3G Method
The HF/STO-3G was particularly useful for looking at the orbitals in the molecule
HOMO orbital
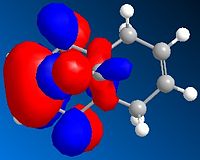
HOMO-1 Orbital
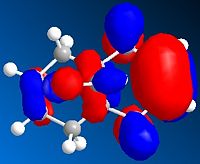
LUMO Orbital
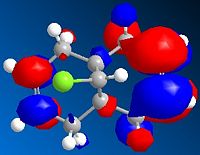
LUMO+1 Orbital
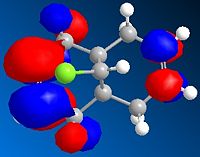
LUMO+2 Orbital
 These orbitals show that the HOMO has its orbital centered around the ring nearest the chlorine. This is because the chlorine is electron withdrawing and so will draw the electrons towards that side of the molecule. The orbital in the LUMO is away from the chlorine, as this is a the lowest orbital with no electrons and so will be away from the area of high electron density (around the chlorine)
These orbitals show that the HOMO has its orbital centered around the ring nearest the chlorine. This is because the chlorine is electron withdrawing and so will draw the electrons towards that side of the molecule. The orbital in the LUMO is away from the chlorine, as this is a the lowest orbital with no electrons and so will be away from the area of high electron density (around the chlorine)
B3LYP Method
The B3LYP method was used to look at the vibrational modes of dichlorocarbene and monochlorocarbene, in particular the stretches for C-Cl and C=C. For dichlorocarbene there will be two C=C stretches referring to the two double bonds and for monochlorocarbene just the one stretch as there is only one double bond.
Vibrational Modes
| Dichlorocarbene | Monochlorocarbene | |||||
| Stretch | Energy level | Wavelength | Absorbance | Energy level | Wavelength | Absorbance |
| C-Cl | 19 | 772.634 | 25.2417 | 19 | 772.634 | 25.2417 |
| C=C | 55 | 1740.75 | 4.1445 | |||
| C=C | 56 | 1760.91 | 3.9037 | 60 | 1758.07 | 4.3505 |
MM2 method
MM2 Method |
HF Method
HF Method |
B3LYP Method
B3LYP Method |
The geometries of the dichlorocarbene vary slightly for the different methods of optimisation. As the optimisation gets more quantum mechanical, the two cyclohexene rings become less planar and more of the boat form. The Carbon-Carbon-Chlorine hydrogen bond was at a very similar angle for the MM2 and B3LYP methods- about 122°, whereas for the HF method this was only 117°. This is suprising as if there was a change in angle I would have expected it to change over the three methods. The length of the Carbon-chlorine bond was lower for the MM2 method- 1.76 A as opposed to a length of about 1.797 for the other two methods. This is probably because the less accurate MM2 method did not optimise the molecule as well.
Miniproject: Determination of Cis and Trans Oak Lactones
It has been found that the two diastereoisomers of lactones from oak barrels can be synthesised using 1,2-dioxine.[1]. This oak lactone is produced naturally within oak barrels used for wine production, which isomer is produced can affect the aroma and taste of the wine so it is important to know which is produced. The two diasteroisomers can be determined using spectroscopic techniques as the groups within the molecule will be in difficult environments in the cis and trans forms.
The oak lactone structure is shown below
Trans oak lactone |
Cis oak lactone |
The two substituent groups that will vary in the isomer are the methyl and butane groups, the carbons on these will be in different environments depending on their geometry. I will also examine the IR spectrum as these may also vary due to the position of these groups.
Which isomer is made by which reaction can be determined by looking at the 13C NMR. The NMR was generated again using the 6-31G base set but this time using DFT method and the mpw1pw91 hybrid functional.
Trans Isomer
NMR Spectrum
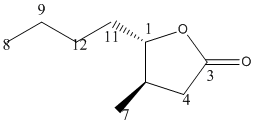
13C NMR (75.5 MHz, CDCl3) = 178.83 (C3), 84.0726 (C1), 46.2702 (C11), 41.2298 (C5), 40.7258, 37.1976, 22.4 (C10,4,9), 17.5 (C7), 13.8 (C8). The results for the trans isomer[2] were closer to the literature values.[3] , probably because the geometric configuration was closer to the real configuration. They weren't exactly the same though, this could be because, as the lactone ring isn't planar. The two different forms of the trans molecule would give slightly different shifts as one is closer to the O-C=O group than the other.
Cis Isomer
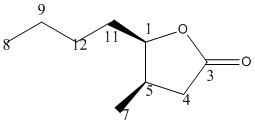
13C NMR (75.5 MHz, CDCl3) δ= 172.782 (C3), 82.5605 (C1), 41.2298 (C5), 40.2218 (C4), 37.1976, 30.6452, 24.5968 (C11,10,9), 19.5565 (C7), 18.0444 (C8).
These results[4] were close to the literature valuesfor the groups in the lactone but were too high for the butane. This is probably due to the conformation calculated using the DFT method. As can be seen on the 3D model, the two sustituent groups are not quite cis; they are on slightly different planes.
Generally, the trans isomer gave greater shifts, suggesting that it was more shielded. This could be because the methyl and butane groups are closer to the C=O and lactone ring and hence will be shielded by the oxygens. Whereas in the cis form, those substiuent groups are more equitorial and hence further away from the O-C=O group not having as much shielding.
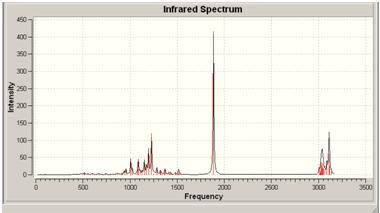
| Energy | wavelength | Absorbance | Stretch |
|---|---|---|---|
| 27 | 1007 | 44.6156 | C-O stretch |
| 30 | 1082 | 15.1 | ring stretch |
| 36 | 1195 | 72.5984 | ring stretch |
| 37 | 1228 | 120.2 | other C-O stretch |
| 59 | 1885 | 416 | C=O stretch |
| 64 | 3033 | 26.9678 | C-H stretch |
| 74 | 3118 | 24.7 | methyl C-H stretch |
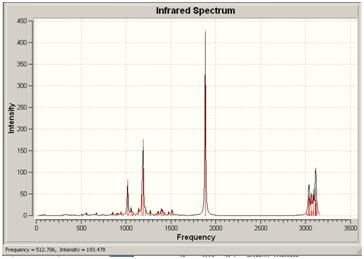
| Energy | wavelength | Absorbance | Stretch |
|---|---|---|---|
| 27 | 1018 | 82.7279 | ring stretch |
| 36 | 1190 | 176.2 | C-O stretch |
| 59 | 1882 | 427 | C=O stretch |
| 63 | 3038 | 38.675 | methyl at end of butane |
| 72 | 3114 | 36.27 | methyl C-H |
The cis molecule has a C-O stretch (for the lactone ring) at 1007, whereas that stretch in the trans molecule can be found at 1190. In the cis lactone, the C-O bond is nearer the substituent groups and so this could affect the frequency of that stretch, making it lower.
Conclusion The NMR was used to determine which isomer was which, as the trans isomer was expected to show greater shielding due to being nearer the C=O bond and the oxygen within the lactone ring.
References
- ↑ [http://pubs.acs.org/doi/pdf/10.1021/ol052754u Utilization of a 1,2-Dioxine for the Synthesis of the Four Possible Stereoisomers of Oak Lactone,Rachel C Brown, Dennis K Taylor and Gordon M Elsey,Organic Letters,2006, Vol 8,3,463]
- ↑ DOI:10042/to-1227
- ↑ [http://pubs.acs.org/doi/suppl/10.1021/ol052754u/suppl_file/ol052754usi20051219_065723.pdf Supporting data for Utilization of a 1,2-Dioxine for the Synthesis of the Four Possible Stereoisomers of Oak Lactone,Rachel C Brown, Dennis K Taylor and Gordon M Elsey,Organic Letters,2006, Vol 8,3,463]
- ↑ DOI:10042/to-1170