Rep:Mod:lh1002
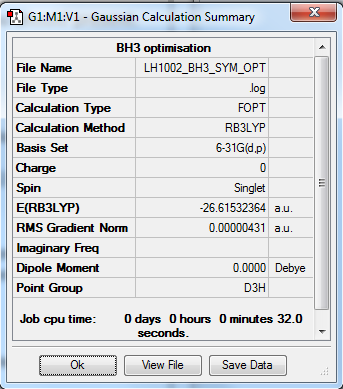
BH3 molecule
Optimization summary
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000034 0.001800 YES RMS Displacement 0.000022 0.001200 YES Predicted change in Energy=-4.417971D-10
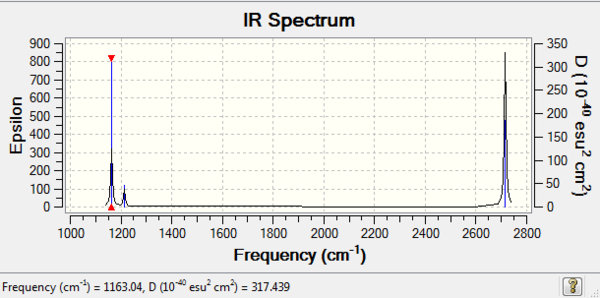
Low frequencies --- -0.6727 -0.3834 -0.0054 12.9097 16.4795 16.4932 Low frequencies --- 1163.0392 1213.2115 1213.2142
vibration modes frequency(cm-1) Intensity Symmetry IR active? Type 1 1163 93 A2" yes out-of-plane bend 2 1213 14 E' slightly bend 3 1213 14 E' slightly bend 4 2582 0 A1' no symmetric stretch 5 2715 126 E' yes asymmetric stretch 6 2715 126 E' yes asymmetric stretch
Despite there are 6 vibration modes, only 3 peaks are observable. Because mode2 and mode3 as well as mode5 and mode6 have the same frequencies, their peaks coincide. Mode4 is IR active because its dipole moment can cancel out.
optimized BH3 molecule |
The completed BH3 frequency *.log file is liked to here
This is the another link if you can't open the link above File:LH1002 BH3 FREQ.LOG
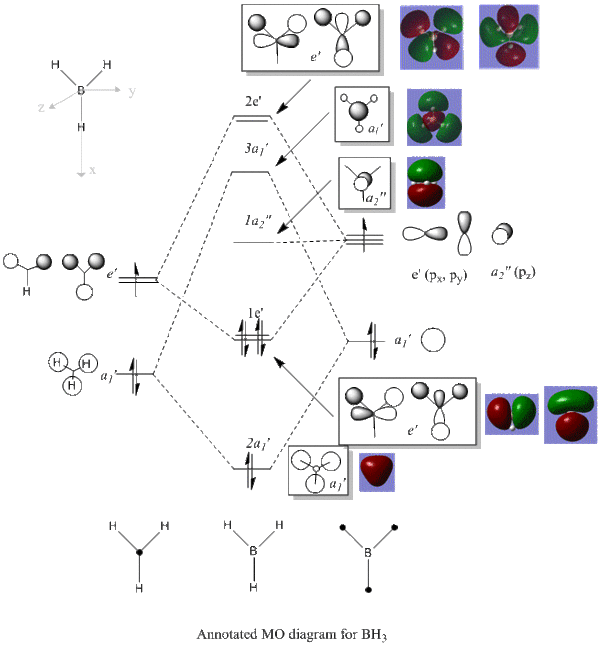
MO diagram
MO QUESTION: 1. Are there any significant differences between the real and LCAO MOs? no 2. What does this say about the accuracy and usefulness of qualitative MO theory? Quite high accuracy Since BH3 is made up by small atoms
Ng611 (talk) 23:30, 15 May 2018 (BST)What about the usefullnes of qMO theory? Are there any differences?
Association energies: Ammonia-Borane
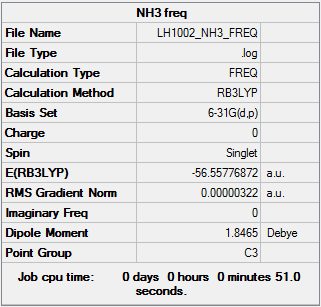
NH3 information
optimized NH3 molecule |
The completed BH3 frequency *.log file is liked to here
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES Predicted change in Energy=-1.131567D-10
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Ammonia-Borane information
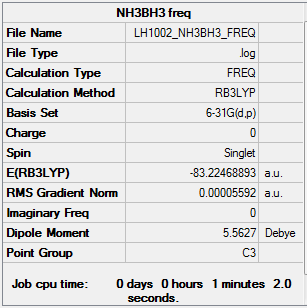
optimized NH3BH3 molecule |
The completed BH3 frequency *.log file is liked to here
Item Value Threshold Converged? Maximum Force 0.000208 0.000450 YES RMS Force 0.000056 0.000300 YES Maximum Displacement 0.001051 0.001800 YES RMS Displacement 0.000430 0.001200 YES Predicted change in Energy=-1.565485D-07
Low frequencies --- -19.3099 -0.0315 -0.0057 0.0256 9.2849 9.2934 Low frequencies --- 262.4242 631.2240 637.8386
Ammonia-Borane association energy
E(NH3)= -56.55777 a.u.= -148464 kJ/mol E(BH3)= -26.61532 a.u.= -69865 kJ/mol E(NH3BH3)= -83.22469 a.u.= -218465 kJ/mol ΔE= E(NH3BH3)-[E(NH3)+E(BH3)] = -218465 -(-69865-148464)=-135 kJ/mol
Association energy is negative because this is the energy released due to bond forming. B-N dative bond is weak cmopared to C-C bond strength which is 376 kJ/mol.
Ng611 (talk) 23:29, 15 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper)
BBr3
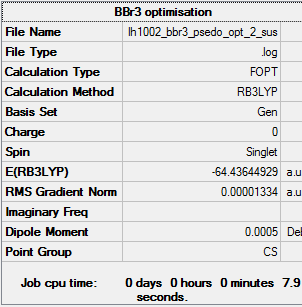
Optimization calculation summary
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000015 0.000300 YES Maximum Displacement 0.000125 0.001800 YES RMS Displacement 0.000078 0.001200 YES Predicted change in Energy=-3.579173D-09
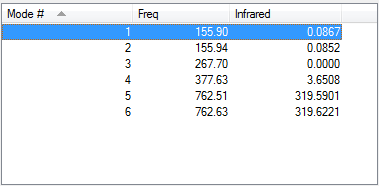
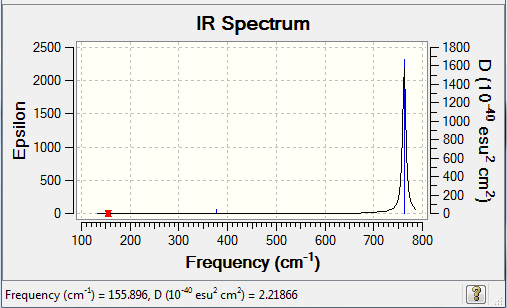
Frequency summary
Low frequencies --- -4.9432 0.0001 0.0001 0.0001 2.2347 3.6178 Low frequencies --- 155.8962 155.9441 267.6992
The completed BH3 frequency *.log file is liked to here
Frequency analysis log file is linked here DOI:10042/202337
optimized BBr3 molecule |
Al2Cl4Br2
five isomers and their symmetry
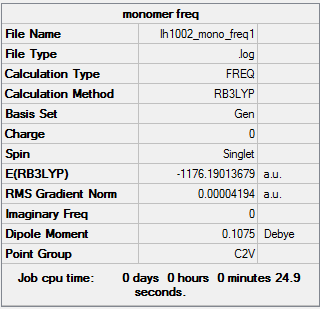
Monomer AlCl2Br
optimized Monomer AlCl2Br |
The completed AlCl2Br frequency *.log file is liked to here
Frequency analysis log file is linked here DOI:10042/202353
Item Value Threshold Converged? Maximum Force 0.000081 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001588 0.001800 YES RMS Displacement 0.000974 0.001200 YES Predicted change in Energy=-1.810813D-07
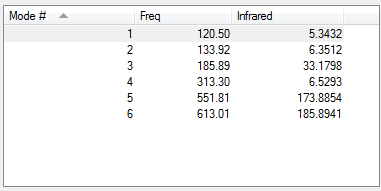
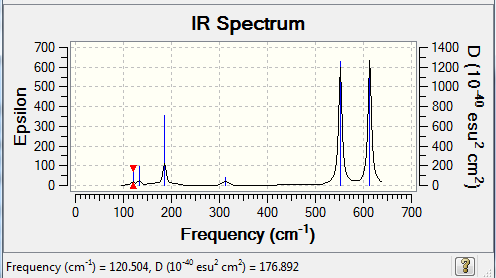
Low frequencies --- 0.0023 0.0035 0.0043 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
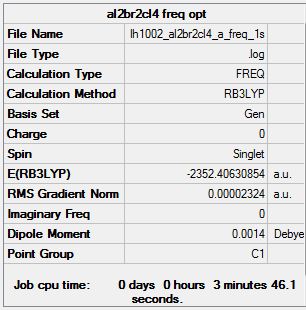
Isomer A with 2 bridging ions information
optimized Isomer A |
The completed BH3 frequency *.log file is liked to here
Frequency analysis log file is linked here DOI:10042/202348
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.001141 0.001800 YES RMS Displacement 0.000499 0.001200 YES Predicted change in Energy=-7.518420D-08
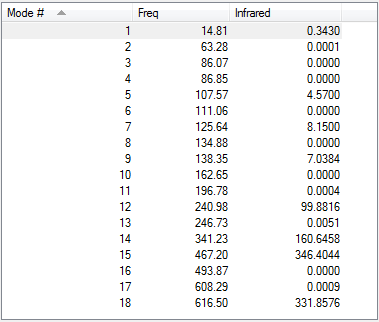
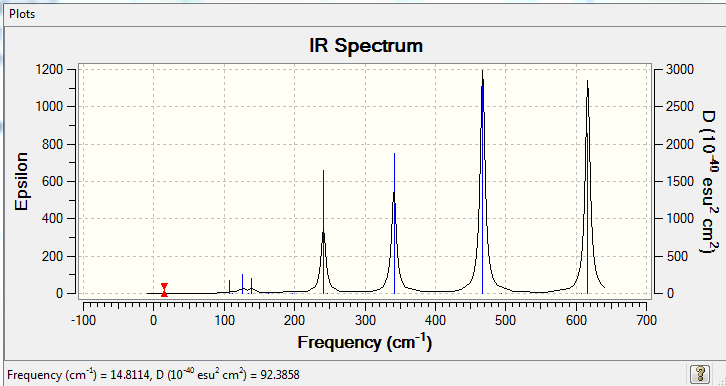
Low frequencies --- -5.1730 -4.9021 -3.0082 -0.0013 0.0012 0.0013 Low frequencies --- 14.8117 63.2813 86.0726
Isomer B with trans terminal Br and bridging Cl
optimized Isomer B |
The completed BH3 frequency *.log file is liked to here
Frequency analysis log file is linked here DOI:10042/202349
Item Value Threshold Converged? Maximum Force 0.000040 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.001356 0.001800 YES RMS Displacement 0.000593 0.001200 YES Predicted change in Energy=-1.805358D-08
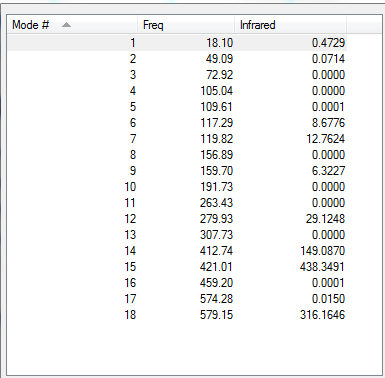
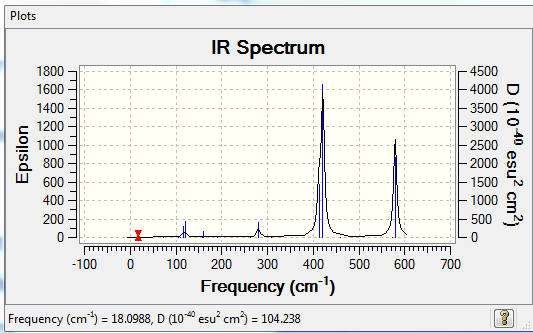
Low frequencies --- -0.0032 -0.0030 -0.0025 1.8920 1.9704 3.9624 Low frequencies --- 18.0988 49.0858 72.9223
Questions about isomers
1.determine the relative energy of these isomers in kJ/mol?
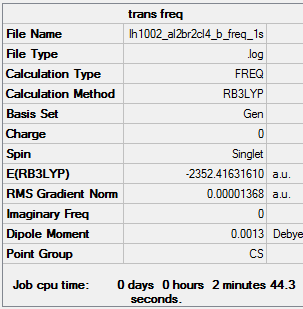
E(a)= -2352.40631 a.u.= -6175066 kJ/mol E(b)= -2352.41632 a.u.= -6175092 kJ/mol E(a-b)= 26 kJ/mol 1 mole of Isomer A has 26 kJ more than 1 mole of isomer B.
2.discuss the relative stability of these conformers with respect to the bridging ions?
Isomer A is less stable than Isomer B because isomer B has bridging Br atoms which are lager with better orbital overlap.
3.determine the dissociation energy for the lowest energy conformer into 2AlCl2Br?
E(monomer)= -1176.19014 a.u.= -3087499 kJ/mol E(b)= -2352.41632 a.u.= -6175092 kJ/mol E(dissociation)= -3087499*2-(-6175092)= 93 kJ/mol
4.is the product more or less stable than the isolated monomers?
The product dimer is more stable since heat will be released if bond is forming.
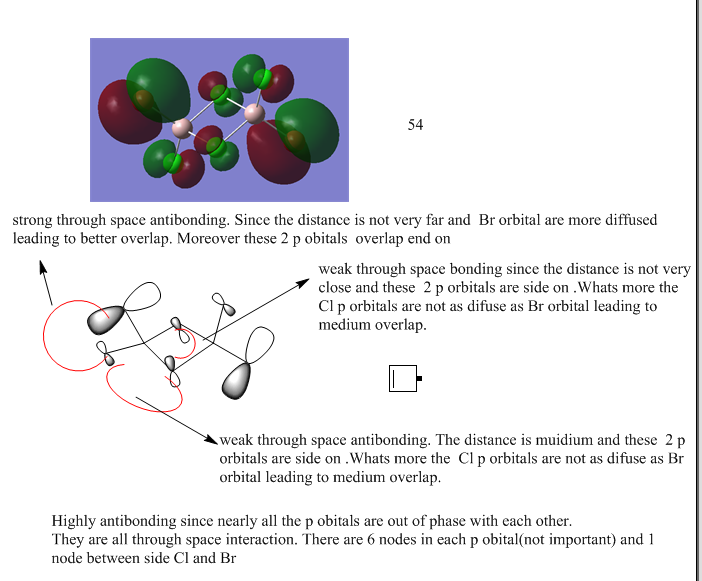
MOs of isomer B
Ng611 (talk) 23:36, 15 May 2018 (BST) You needed to look at 3 MOs as opposed to just one. You should also aim to format your MO analysis in the style that you learned in your MO course. The orbital that you've analysed is correct however, well done.
Ng611 (talk) 23:38, 15 May 2018 (BST) A good report overall. Excellent first section. You let yourself down with your MO analysis in the second section (you needed to analyse more than 1 MO) as well as with a few minor errors here and there, but otherwise good.