Rep:Mod:lg1109mod1
Module 1: Structure and Spectroscopy
Part 1.1: The Hydrogenation of Cyclopentadiene Dimer
Dimerisation
Cyclopentadiene is a colourless liquid cyclic alkene. It can undergo [4+2] cycloaddition with itself to form a dimer at room temperature. One molecule of cyclopentadiene acts as a 4п-electron diene and the other acts as a 2п-electron dieneophile. The obtained dicyclopentadiene is a Diels-Alder adduct. The dimer exists in two conformational isomers, either exo- or endo-form. The predomination form of the products is determined by taking into account both the kinetic and thermodynamic factors of the reaction.
The two isomers are drawn by ChemBio3D and their geometries are optimised with the MM2 force field option to minimize the energy of the system. This code allows us to calculate various interactions of the molecule including bond stretch, bends, torsions, van der Waals repulsions and electrostatic attractions. All of those interactions sum up to give a measurement of “Total Energy”. In order to compare the two isomers, the differences of each interaction are calculated and listed in the table.
| Energy Type | Exo- Form (kcal/mol) | Endo- Form (kcal/mol) | Difference (kcal/mol) |
|---|---|---|---|
| Stretch | 1.29 | 1.25 | 0.04 |
| Bend | 20.58 | 20.85 | -0.27 |
| Stretch-Bend | -0.84 | -0.84 | 0.00 |
| Torsion | 7.66 | 9.51 | -1.85 |
| Non-1,4 VDW | -1.42 | -1.54 | 0.12 |
| 1,4 VDW | 4.23 | 4.32 | -0.09 |
| Dipole/Dipole | 0.38 | 0.45 | -0.07 |
| Total Energy | 31.88 | 34.00 | -2.12 |
According to the table, the exo isomer has a slightly lower total energy comparing to the endo- form which means the thermodynamic product is the exo- form. The main difference arise from the torsion energy which is 1.85 kcal/mol smaller for the exo- form. This results from the more favourable staggered conformation along the newly formed C-C bond which release the steric clashes exerted by the two hydrogen atoms, making the exo- product more thermodynamically stable. However, the literature indicates that the endo- product is always the predominant isomer found for this reaction. The explanation for this observation is that the reaction favours the kinetic product which formed under kinetic control. The reaction pathway with lower activation energy proceeds faster and hence the endo- product forms more easily at room temperature. On the other hand, the exo- product will be formed if the reaction is under thermodynamic control which occurs at higher temperature with relative long reaction time.
Hydrogenation

The endo- dicyclopendiene can be undergo hydrogenation to give two possible structures shown on the left, mainly depending on which of the two C=C bonds is hydrogenated.
As before, Molecule 3 and Molecule 4 are imported by ChemBio 3D and MM2 calculations were run in order to produce the most stable configurations and to determine which of the two forms is more likely to form. The obtained results are summarized in the table below.
| Energy Type | Exo- Form (kcal/mol) | Endo- Form (kcal/mol) | Difference (kcal/mol) |
|---|---|---|---|
| Stretch | 1.23 | 1.13 | 0.1 |
| Bend | 18.77 | 13.01 | 5.76 |
| Stretch-Bend | -0.75 | -0.57 | -0.18 |
| Torsion | 12.73 | 12.41 | 0.32 |
| Non-1,4 VDW | -1.34 | -1.32 | -0.02 |
| 1,4 VDW | 6.05 | 4.44 | 1.61 |
| Dipole/Dipole | 0.16 | 0.14 | 0.02 |
| Total Energy | 36.86 | 29.25 | 7.61 |
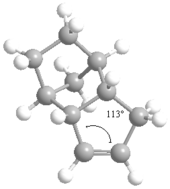
According to the table, Molecule 4 is more thermodynamically stable as its total energy is lower than Molecule 3 by 7.61 kcal/mol. The energy bending interaction, torsions and 1, 4 Van der Waals contribute the most to this difference. The measurements of the dihedral angles between the C=C and C-H show that the value turns out to be 107° for Molecule 3 and 113° for Molecule 4. The deviation of bond angles from the ideal value will destabilise the energy of molecule due to the excess potential energy is accumulated in strain. The alkene bonds are sp3 hybridized so that the ideal bond angle around the carbon centre is 120°. Molecule 4 has the value of bond angle closer to the ideal value in comparison to Molecule 3, hence contains less angular strain, making it thermodynamically more stable.

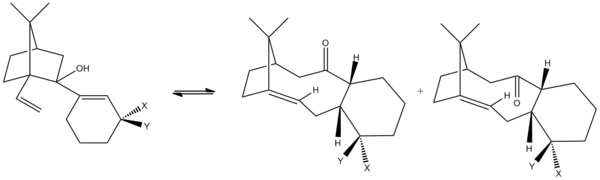
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
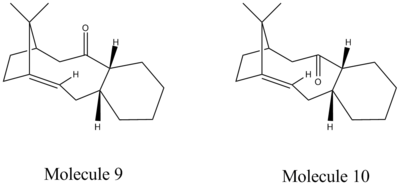
Taxol is an anti-cancer chemotherapy drug especially in the treatment of breaset, ovarian, lung, bladder, prostate, as well as other types of solid tumor cancers and Kaposi's sarcoma.[1] It is derived from the bark of the Pacific yew tree and was brought into research in the early 1960's. Paquette proposed two key intermediates from the rearrangement of the oxy-Cope in the synthesis of taxol.[2] The carbiniols here undergoes atroposelective anionic oxy-Cope rearrangements to form the alternative carbonyl isomers shown in the figure where X=Y=H. The two isomers differ from each other by the position of the carbonyl group. Molecule 9 has the C=O points upwards whereas Molecule 10 has the C=O points downwards with the cyclohexane ring structure locked.[3]
The structures of both intermediates are produced using ChemDraw as below.[4]
The two isomers are then drawn by ChemBio3D and the geometries are optimised with the MM2 force field option to obtain the most energetically stable conformation. Cyclohexane ring parts of both isomers were produced in chair conformation as it is the lowest energetic form. This was done by minimizing the energy using MM2 first, followed by rearranging the structures to chair manually as the first obtained molecules were in twist boat conformation. The second MM2 calculation was then carried out. This is necessary because the twist boat conformation for both isomers is generally about 6 kcal/mol greater in energy. The results are illustrated in the table below.
| Energy Type | Exo- Form (kcal/mol) | Endo- Form (kcal/mol) | Difference (kcal/mol) |
|---|---|---|---|
| Stretch | 2.78 | 2.61 | 0.17 |
| Bend | 16.54 | 11.34 | 5.20 |
| Stretch-Bend | 0.43 | 0.34 | 0.09 |
| Torsion | 18.26 | 19.66 | -1.4 |
| Non-1,4 VDW | -1.55 | -2.15 | 0.6 |
| 1,4 VDW | 13.11 | 12.87 | 0.24 |
| Dipole/Dipole | -1.72 | -2.00 | 0.28 |
| Total Energy | 47.84 | 42.68 | 5.16 |
The difference of the total energy is due to the bend interaction which refers to the energy associated with the displacement of any bond angle from equilibrium positions. This mainly results from the greater steric hindrance between the carbonyl group and the hydrogens in the axial positions for isomer 9.[5]
Merck Molecular Force Field (MMFF) is a set of force fields which based on the MM3 force field developed by Merck Research Laboratories. They perform particularly well for a wide range of organic chemistry calculations. MMFF94 is the first published force field within the family .[6] The total energy of the intermediates are also calculated by MMFF94 which listed in the table below.
| Energy Type | Molecule 9 (kcal/mol) | Molecule 10 (kcal/mol) | Difference (kcal/mol) |
|---|---|---|---|
| Total Energy | 70.54 | 60.56 | 9.98 |
MMFF94 mainly differs from the MM2 calculations in the analysis of non-bonded interactions of the molecules. The results show that MM2 energies are about 20 kcal/mol lower than the MMFF94 ones. However, the general trend is retained. Therefore, both of the force field calculations proof that Molecule 10 is the thermodynamic more stable intermediate instead of its carbonyl isomer 9.[7] The total energy of the intermediates are also calculated by MMFF94 which listed in the table below.
When Molecule 10 is hydrogenated, the unsaturated carbon-carbon double bonds is hydrolysized to give a single bond which is represented by the structure shown below.

The MM2 calculation was performed on the hydrogenated molecule to minimize the energy.[8]
| Energy Type | Hydrogenated Derivative(kcal/mol) |
|---|---|
| Stretch | 3.02 |
| Bend | 15.20 |
| Stretch-Bend | 0.59 |
| Torsion | 21.61 |
| Non-1,4 VDW | -1.50 |
| 1,4 VDW | 15.61 |
| Dipole/Dipole | -1.73 |
| Total Energy | 52.80 |
The total energy of the fully saturated molecule are slightly higher than the alkene 10. During the hydrogenation, the two newly added hydrogen atoms exerts steric clash to the ring structure which results the main increase in the bending interactions.
Regioselective Addition of Dichlorocarbene
The structure of dichlorocarbene is first produced using ChemDraw as below.The molecule can also be examed by 3D version.
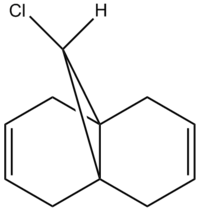
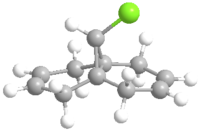
The molecule was then imported to ChemBio 3D and MM2 calculation was run to roughly minimizing the geometric energy. The MM2 calculation results are listed below.
| Energy Type | Molecule 12(kcal/mol) |
|---|---|
| Stretch | 0.62 |
| Bend | 4.74 |
| Stretch-Bend | 0.04 |
| Torsion | 7.66 |
| Non-1,4 VDW | -1.07 |
| 1,4 VDW | 5.79 |
| Dipole/Dipole | 0.11 |
| Total Energy | 17.89 |
This is then preceded by MOPAC/PM6 to produce the valence-electron molecular wavefunction and molecular orbitals as the close examine of the reactive orbitals will give lots of information about the olefin reactions. HOMO molecular orbitals together with HOMO-1, LUMO, LUMO+1 and LUMO+2 are all investigated. The positive part of the orbital is represented in red colour while the negative orbital is in blue colour.
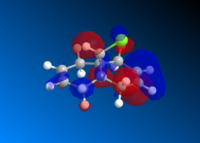
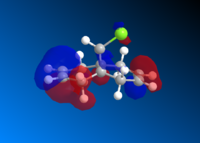
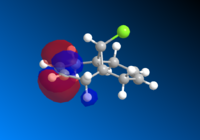


The molecule has a point group of Cs which is generally symmetrical regarding the plane σh. The exo- alkene is more stabilised comparing to the endo HOMO π-orbital because of the antiperiplanar overlap of the σ anti-bonding orbital of the C-Cl bond. The low-lying *σ C-Cl orbital acts as a good electron acceptor because of the large electronegativity of Cl.[9]
The monosubstituted alkene is also operated in the same way.

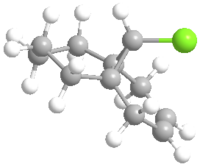
The molecule was then imported to ChemBio 3D and MM2 calculation was run to roughly minimizing the geometric energy. The MM2 calculation results are listed below.
| Energy Type | Molecule 13(kcal/mol) |
|---|---|
| Stretch | 0.90 |
| Bend | 4.69 |
| Stretch-Bend | 0.01 |
| Torsion | 10.76 |
| Non-1,4 VDW | -1.06 |
| 1,4 VDW | 6.97 |
| Dipole/Dipole | 0.07 |
| Total Energy | 22.34 |
The prediction of infrared spectra is also done for Molecule 12 and Molecule 13 by submitting the molecules to supercomputer to allow the calculations.

The out IR file is introduced to GaussView 5.0. It is found that the C-Cl bond produce a peak at 770.8 cm-1 due to bond stretching. The stretch at 1737.1 cm-1 corresponds to the exo- double bond while the stretch at 1757.4 cm-1 corresponds to the endo- double bond form. By comparing the frequencies of the two forms, it can be seen that the exo- double bond is weaker as the wavenumber is lower than of the endo-.
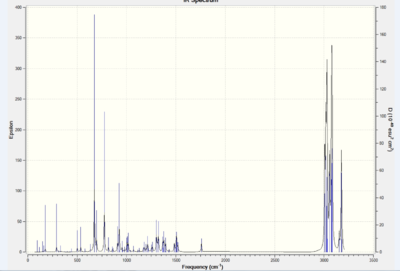
GaussView 5.0 is again used here to analyse the IR spectrum obtained. It is found that C-Cl stretch occurs at 775.0 cm-1 and the endo- double bonds at 1758.1 cm-1. The main difference is the lack of the exo- double bond as well as the shift of the C-Cl frequency for this monosubstituted alkene. This can be explained by lacking of electron density donation to the C-Cl anti-bonding orbital, so that bond order is retained as well as the bond strength.
Monosaccharide chemistry: glycosidation

Fischer glycosidation usually involves neighbouring group participation. The -OAc group takes part in the formation of the alpha and beta anomers as the X group indicated in the figure is replaced by a nucleophile. The intermediate step shows that the lone pair on the oxygen of the acetyl group attacks the position which would be substituted so that the ring conformation is locked. The nucleophile now can only attack either from the top or the bottom face of the ring structure. [10] The mechanism is shown below.

In order to analyse the molecules with minimal computational demand, a methyl group has been chosen to represent the R group. The choice preserves the information contained by the molecules while making the time of calculation much shorter. Ring A has the acetyl group in the equatorial position while Ring B has the acetyl group in the axial position. Ring A' is obtained by rotating the acetyl group around the C-O bond so that the C=O on the acetyl group are forced away from the oxonium atom in the ring. Ring B' is obtained by similar manipulation. Therefore, Ring A' and Ring B' can be regarded as the ring-flipped conformer of the corresponding A and B.
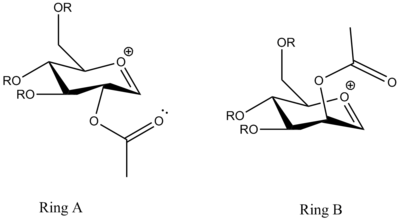
The MM2 and MOPAC/PM6 calculations are run for all the four molecules in order to completely optimise the energy of the molecules. The obtained results are summarized in the table below.
| Energy Type | Ring A (kcal/mol) | Ring A' (kcal/mol) | Ring B (kcal/mol) | Ring B'(kcal/mol) |
|---|---|---|---|---|
| Stretch | 2.73 | 2.34 | 2.46 | 2.23 |
| Bend | 14.69 | 11.64 | 11.54 | 10.03 |
| Stretch-Bend | 1.10 | 0.89 | 0.84 | 0.83 |
| Torsion | 2.01 | 3.05 | 1.18 | 1.88 |
| Non-1,4 VDW | -0.32 | -1.88 | -0.58 | -4.25 |
| 1,4 VDW | 18.65 | 19.31 | 19.30 | 19.24 |
| Charge/Dipole | -16.75 | -15.56 | -17.00 | -2.54 |
| Dipole/Dipole | 6.01 | 4.46 | 5.97 | 4.16 |
| Total Energy | 28.11 | 24.25 | 23.70 | 31.56 |
| MOPAC/PM6 | -91.66 | -77.40 | -77.31 | -69.36 |
The acetyl group in Ring A is point below the flat oxonium ring structure so that the incoming nucleophile is preferred to attack the top face. It is found that the MOPAC/PM6 heats of formation of Ring C is almost the same as of Ring A, which both have the value of -91.7 kcal/mol. In contrast, the acetyl group in Ring C is point upwards the oxonium ring so that the nucleophile can attack from the bottom face.
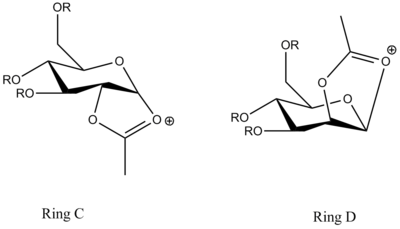
| Energy Type | Ring C (kcal/mol) | Ring C' (kcal/mol) | Ring D (kcal/mol) | Ring D'(kcal/mol) |
|---|---|---|---|---|
| Stretch | 1.76 | 2.71 | 1.85 | 3.00 |
| Bend | 16.38 | 17.47 | 14.42 | 18.07 |
| Stretch-Bend | 0.73 | 0.80 | 0.60 | 0.89 |
| Torsion | 7.62 | 8.17 | 7.66 | 9.41 |
| Non-1,4 VDW | -4.09 | -2.43 | -5.13 | -3.03 |
| 1,4 VDW | 17.45 | 19.40 | 18.51 | 19.31 |
| Charge/Dipole | -4.04 | -0.33 | 2.55 | -0.75 |
| Dipole/Dipole | -0.95 | -1.75 | -0.17 | -1.04 |
| Total Energy | 34.86 | 44.03 | 40.29 | 45.86 |
| MOPAC/PM6 | -91.65 | -66.87 | -85.93 | -66.65 |
MINI Project: The total synthesis of (-)Cubebol

| Energy Type | MM2 Total Energy (kcal/mol) | MOPAC/PM6 Total Energy(kcal/mol) |
|---|---|---|
| Molecule 11 | 11.83 | -80.76 |
| Molecule 17 | 101.63 | -45.78 |
| Molecule 18 | 35.56 | -75.43 |
| Molecule 1 | 34.12 | -82.83 |

| Assignment | Predicted freq. (cm-1) | Literature freq. (cm-1) | Difference % |
|---|---|---|---|
| Alkyl C-H stretching (symmertic) | 2984 | 2961 | 0.78 |
| Alkyl C-H stretching (assymmertic) | n/a | 2931 | n/a |
| C-H stretching | n/a | 2872 | n/a |
| C=O | 1802 | 1712 | 5.26 |
| C-H bending | 1434 | 1455 | 1.44 |
| C-H rocking | 1372 | 1369 | 0.22 |
| C-H wagging | 1308 | 1315 | 0.53 |
| C-H out of plane bending | 754 | 750 | 0.57 |
| Assignment | Predicted freq. (cm-1) | Literature freq. (cm-1) | Difference % |
|---|---|---|---|
| Alkyl C-H stretching (symmertic) | 2984 | 2955 | 0.98 |
| Alkyl C-H stretching (assymmertic) | n/a | 2929 | n/a |
| C-H stretching | n/a | 2851 | n/a |
| C-H bending | 1453 | 1463 | 0.68 |
| C-H rocking | 1373 | 1367 | 0.43 |
| C-H wagging | 909 | 918 | 0.98 |
| C-H out of plane bending | 858 | 836 | 2.6 |


| Assignment | Predicted freq. (cm-1) | Literature freq. (cm-1) | Difference % |
|---|---|---|---|
| O-H Stretching | 3795 | 3608 | 5.18 |
| Alkyl C-H stretching | 2989 | 2958 | 1.05 |
| Alkyl C-H stretching (symmertic) | n/a | 2955 | n/a |
| n/a | n/a | 2928 | n/a |
| n/a | n/a | 2851 | n/a |
| n/a | n/a | 2870 | n/a |
| C-H bending | 1386 | 1385 | 0.07 |
| C-H rocking | 1370 | 1371 | 0.07 |
| C-H wagging | 1225 | 1216 | 0.74 |
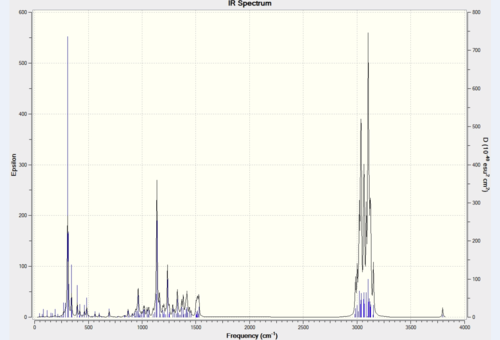
| Assignment | Predicted freq. (cm-1) | Literature freq. (cm-1) | Difference % |
|---|---|---|---|
| O-H stretching | 3152 | 3350 | 5.91 |
| Alkyl C-H stretching | n/a | 2951 | n/a |
| n/a | n/a | 2860 | n/a |
| C-H bending | 1500 | 1490 | 0.67 |
| C-H rocking | 1138 | 1142 | 0.35 |
| C-H wagging | 910 | 910 | 0.00 |
Most of the predicted IR spectra above have good consistency with the literature values. Only a few peaks are missing. This confirms that the computational calculations for IR spectra are accurate and reasonable.
References
- ↑ 1.Developmental Therapeutics ProgramDOI:http://dtp.nci.nih.gov/timeline/flash/success_stories/S2_Taxol.htm
- ↑ 2.S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319DOI:http://www.sciencedirect.com/science/article/pii/S0040403900926170
- ↑ 3.See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 DOI:http://pubs.acs.org/doi/abs/10.1021/ja00824a025
- ↑ 4.Vancomycin: J. Am. Chem. Soc., 1999, 121, 3226.DOI:http://pubs.acs.org/doi/abs/10.1021/ja990189i
- ↑ 5.DOI:http://mason.gmu.edu/~aphan6/projects/protein/PART%20III.htm
- ↑ 6.DOI:http://en.wikipedia.org/wiki/Merck_Molecular_Force_Field
- ↑ 7.Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94, Thomas A. Halgren, J. Comp. Chem.; 1996; 490-519DOI:{{{1}}}
- ↑ 8.Wilhelm F. Maier, Paul Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891.DOI:http://pubs.acs.org/doi/abs/10.1021/ja00398a003
- ↑ 9.B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447.DOI:http://pubs.rsc.org/en/Content/ArticleLanding/1992/P2/p29920000447
- ↑ 10.D. M. Whitfield, T. Nukada, Carbohydr. Res., 2007, 342, 1291.DOI:http://www.sciencedirect.com/science/article/pii/S0008621507001668
- ↑ 11.D. M. Hodgson, S. Saifullah, JOC. Article, 20010, 75, 2157.DOI:http://pubs.acs.org/doi/abs/10.1021/jo9022974
