Rep:Mod:lfc14 table
Appearance
Exercise 1
| IRC | Negative Frequency at Transition State |
|---|---|
|
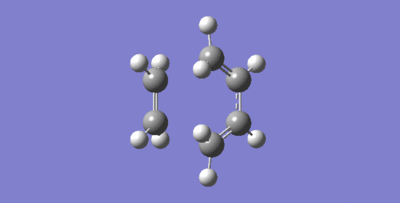
|
| Bond | Reactants / Å | Transition State / Å | Products / Å |
|---|---|---|---|
| C1-C2 | 1.32731 | 1.38177 | 1.53800 |
| C2-C3 | N/A | 2.11466 | 1.53602 |
| C3-C4 | 1.33537 | 1.37979 | 1.49270 |
| C4-C5 | 1.46818 | 1.41097 | 1.33313 |
| C5-C6 | 1.33533 | 1.37979 | 1.49269 |
| C6-C1 | N/A | 2.11457 | 1.53603 |



Exercise 2
| Endo | Exo | |
|---|---|---|
| Reactants / kJmol−1 | -1313771 | -1313771 |
| Transition State / kJmol−1 | -1313621 | -1313614 |
| Products / kJmol−1 | -1313849 | -1313845 |
| Activation Energy / kJmol−1 | 149.6981 | 157.5352 |
| Reaction Energy / kJmol−1 | -77.5205 | -73.9235 |
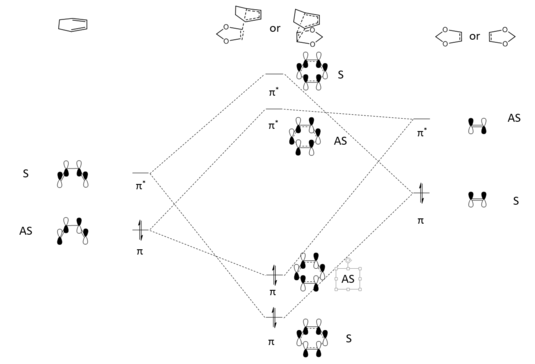

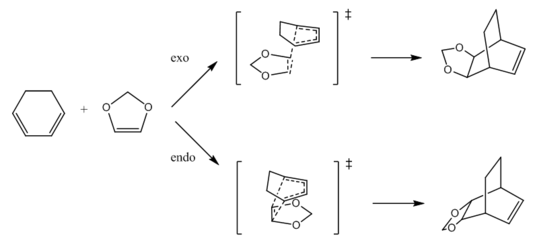
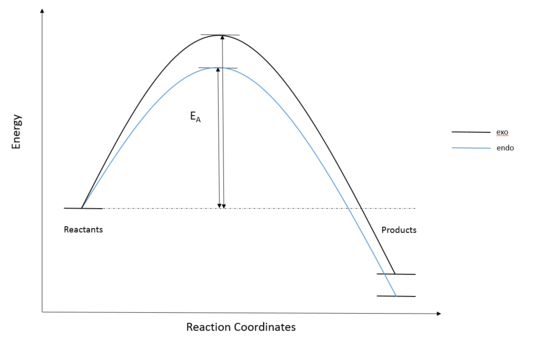
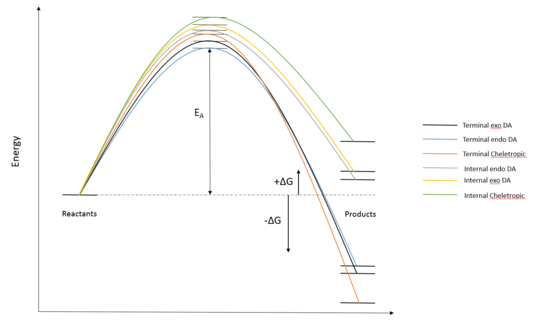
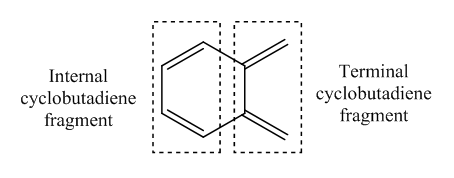
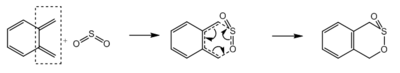
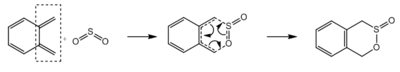
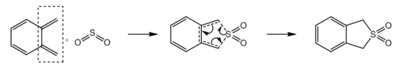
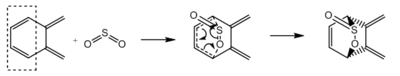
Exercise 3
| Reaction | Diels Alder (exo) | Diels Alder (endo) | Cheletropic | Diels Alder (exo) | Diels Alder (endo) | Cheletropic |
|---|---|---|---|---|---|---|
| Cyclobutadiene fragments | Terminal | Terminal | Terminal | Internal | Internal | Internal |
| Reactants / kJmol−1 | 156.1804 | 156.1804 | 156.1804 | 156.1804 | 156.1804 | 156.1804 |
| Transition State / kJmol−1 | 241.7481 | 237.7652 | 260.0872 | 275.8218 | 267.9847 | 296.6971 |
| Products / kJmol−1 | 56.3275 | 56.9655 | 0.0026 | 176.7039 | 172.2590 | 203.2740 |
| Activation Energy / kJmol−1 | 85.5676 | 81.5848 | 103.9067 | 119.6414 | 111.8042 | 140.5167 |
| Reaction Energy / kJmol−1 | -99.8530 | -99.2150 | -156.1780 | 20.5235 | 16.0786 | 47.0936 |