Rep:Mod:lfc14
Transition state
Introduction
Diels-Alder reaction is an important reaction in organic synthesis. However, Diels-Alder reaction can proceed through different pathways (endo and exo) and sometimes completing pathway can exist. These pathways can be calculated by computational methods to understand the relative rates and stability of products.
To simulate a reaction, the computer considers all possible combination of the atoms and molecules involved and calculates their respective electronic potential energy with respect to all degrees of freedom (normal vibrational modes). There are 3N-5 (where N is the number of atoms) vibrational modes for linear molecules and 3N-6 vibrational modes for non-linear molecules. Varying only two coordinates (degree of freedom) of the system gives a potential energy surface (PES).
In PES, the minima (gradient of zero with positive second derivative along any reaction coordinates) on a PES gives the equilibrium position of the molecules. The minimum energy pathway, usually represented as Intrinsic Reaction Coordinate (IRC), between two minima give the reaction coordinate. The highest energy point (a stationary point with gradient of zero and a negative second derivative along the reaction coordinate) along the reaction coordinate is the transition state. The transition state would have one negative vibrational frequency in vibrational calculations, which indicates the reaction coordinate.
Diels-Alder reaction (exercise 1), inverse electron demand Diels-Alder Reaction (exercise 2), Hetero-Diels-Alder reaction (exercise 3) and Cheletropic reaction (exercise 3) were studied. Semi-empirical PM6 was used to for initial optimisation and while DFT B3LYP at 631g(d) basis set is used for further accuracy. PM6 uses experimental data to shorten calculation procedures while B3LYP uses Density Functional Theory to calculate geometries completely.
Nf710 (talk) 21:03, 21 March 2017 (UTC) Good understanding here. One point is that B3LYP is a hybrid model which uses HF to calculate the exchange correlation.
Excercise 1
(̴̴̴~ *Exercise)
In this exercise, the Diels-Alder reaction between butadiene and ethane was studied. The reactants, transition states and products were optimised at semi-empirical PM6 level.
Molecular Orbital
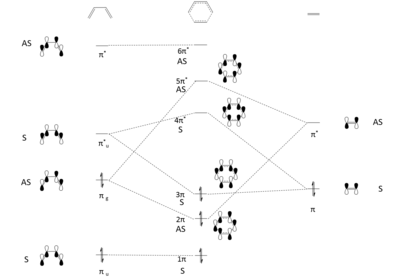
Figure 2. shows the frontier molecular orbitals (LUMO +1, LUMO, HOMO and HOMO – 1) of the transition state with symmetry (symmetric or asymmetric) labelled. Jmol objects of the transition states orbitals (Transition State Jmol) and the HOMO and LUMO of butadiene and ethane were also showed in Butadiene Jmol and Ethene Jmol to visualise how the transition state orbitals were formed. The MO Theory states that only molecular orbitals with same symmetry interact with each other. MO diagram in Figure 2. suggest that asymmetric orbitals interact while symmetric orbitals. (Fv611 (talk) 17:41, 15 March 2017 (UTC) Think you just forgot to finish your sentence. Both AS/AS and S/S interactions are allowed. Also, the correct term is antisymmetric, not asymmetric)
This can be reflected by the MO diagram and the Jmol objects. Therefore from both MO theory and computational simulation, it can be concluded that a symmetric-antisymmetric interaction has an orbital overlap of zero while a symmetric-symmetric interaction and an antisymmetric-antisymmetric interaction has a non-zero orbital overlap.
Bond Length Measurements

| Bond | Reactants / Å | Transition State / Å | Products / Å |
|---|---|---|---|
| C1-C2 | 1.32731 | 1.38177 | 1.53800 |
| C2-C3 | N/A | 2.11466 | 1.53602 |
| C3-C4 | 1.33537 | 1.37979 | 1.49270 |
| C4-C5 | 1.46818 | 1.41097 | 1.33313 |
| C5-C6 | 1.33533 | 1.37979 | 1.49269 |
| C6-C1 | N/A | 2.11457 | 1.53603 |
Change of bond length during reaction was tabulated in Figure 3. and Table 1. The bond length of C3-C-4, C5-C6 and C1-C2 increased from 1.33Å to 1.54Å suggest a change from C=C (1.33Å) to C-C (1.54Å).[1] The bond length of C4-C5 decreased from 1.47Å to 1.33Å suggest a change from C-C (1.54Å) to C=C (1.33Å). The length of C2-C3 and C1-C6 decreased from infinity to 1.54Å suggesting a formation of a C-C bond. At transition state, C2-C3 and C1-C6 bond length were smaller than twice of the van der Waal’s radius (1.70Å)[2] suggesting the overlapping of carbons (interaction). Bond length of C1-C2, C3-C4, C4-C5 and C5-C6 were between the bond length of a C-C and C=C bond, suggesting a partial double bond (hybridisation shifting from sp3 to sp2 or otherwise).
Reaction Mechanism Analysis
| IRC | Negative Frequency at Transition State |
|---|---|
|
|
The reaction proceed via a concerted mechanism, as shown by the IRC and the negative vibration frequency (which indicates the reaction pathway) at the transition state (Table 2). Both carbons on the alkene have the same distance at transition state while both carbons approach the diene carbons at the same time in the negative vibrations, suggesting a synchronous, concerted C-C bonds formation.
Excercise 2
Molecular Orbital
The inverse electron demand Diels Alder reaction was studied in this exercise. The reaction between cyclohexadiene and 1,3 dioxole was simulated. The reactants, products (both exo and endo) and transition states were optimised by DFT B3LYP with 6-31G (d) basis set. According to the MO theory, orbitals of the same symmetry will interact to give two resulting orbitals of same symmetry. In this reaction, the HOMO and LUMO of the transition state are both asymmetric(this can be seen in Endo TS and Exo TS Jmol), which means these orbitals are formed from the interaction of the LUMO of the diene and the HOMO of the dienophile (Figure 5). Energy calculation of the transition states also confirmed the order of the energy levels in the MO diagram. This suggests a reverse electron demand, which could be explained by the electron donating oxygens in 1,3 dioxole, raising the energy levels of the dienophile. The now raised dienophile HOMO has a closer energy level with the diene LUMO while the dienophile LUMO energy level is further apart from the diene HOMO, resulting in the shift of MO energy order.
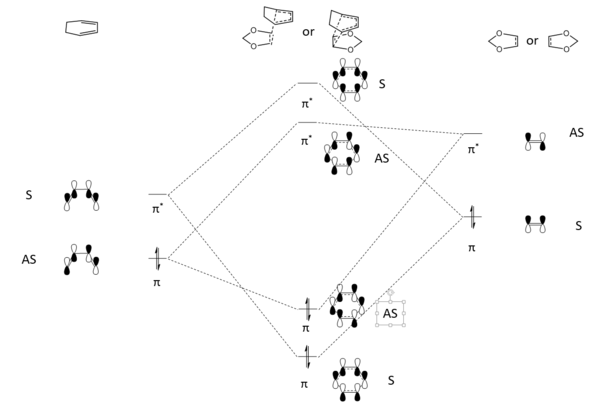
Activation and Reaction Energies
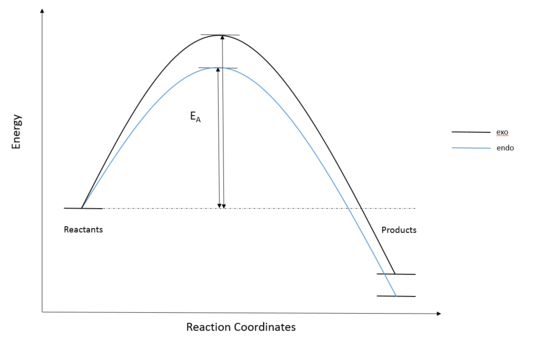
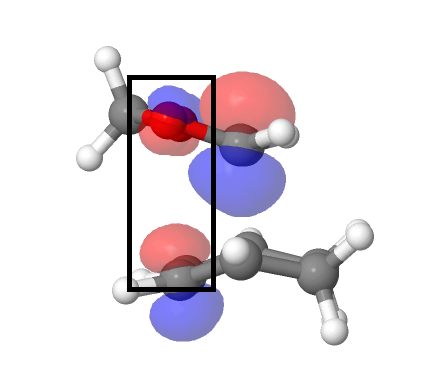
The reaction energies and activation energies of both endo and exo reactions were tabulated in Table 3. The energy of the reactants were calculated by adding the energy of the optimised cyclohexadiene and 1,3 dioxole together, assuming the reactants are infinetly separated at the start of the reaction. In a typical Diels Alder reaction, the endo pathway is kinetically favourable due to secondary orbital interactions (only available to endo TS) between oxygen and diene p orbitals (Figure 7)(Endo TS and Exo TS Jmol). This was reflected by the calculations, where the endo pathway had a lower activation energy. The exo product is more thermodynamically favourable, as the product is less sterically crowed. However, computer calculation from Table 3. showed otherwise, suggesting a more exothermic endo product. This could be explained by the possible steric clash between bridging C-H hydrogens and terminal C-H hydrogen in five-membered ring (Figure 8). Thus the endo pathway was both kinetically and thermodynamically favourable in the inverse electron demand Diels Alder reaction between 1,3 dioxole and cyclohexadiene.
| Endo | Exo | |
|---|---|---|
| Reactants / kJmol−1 | -1313771 | -1313771 |
| Transition State / kJmol−1 | -1313621 | -1313614 |
| Products / kJmol−1 | -1313849 | -1313845 |
| Activation Energy / kJmol−1 | 149.6981 | 157.5352 |
| Reaction Energy / kJmol−1 | -77.5205 | -73.9235 |
 |
 |
 |
Nf710 (talk) 21:12, 21 March 2017 (UTC) Excellent understanding of the SSO and the sterics giving rise to the kenetic and thermo products. You seemed to have caluculated reactants wrong, but the energy of the products and TS are correct
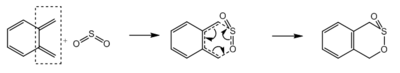
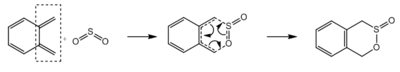
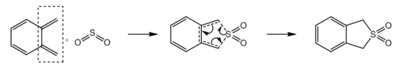
Excercise 3

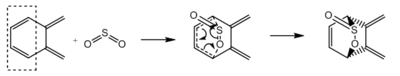
In this exercise, the reaction between o-xylylene and sulphur dioxide is studied. Sulphur dioxide can react with dienes via Hetero-Diels-Alder reaction and Cheletropic reaction, giving three possible products. When sulphur dioxide reacts with o-xylene, which has two cis-butadiene fragments (Figure 9), six possible products can be formed via Hetero-Diels-Alder reaction and Cheletropic reaction (Table 4). All six reactions were simulated at Semi-emperical PM6 level, with IRC, activation energies and reaction energies calculated to evaluate the thermodynamic and kinetic favourability of these reactions.
(Well done getting all IRCs and TSs Tam10 (talk) 10:59, 23 March 2017 (UTC))
Reaction and Activation Energies
Reaction energy and activation energy were tabulated in (Table 5) and reaction coordinates were sketched on (Figure 10) to reflect the relative reaction energies and activation energies. Energies of the reactants were calculated by adding the energy of individual reactant. This effectively assumed both reactants were infinitely separated to at the start of the reaction.
| Reaction | Diels Alder (exo) | Diels Alder (endo) | Cheletropic | Diels Alder (exo) | Diels Alder (endo) | Cheletropic |
|---|---|---|---|---|---|---|
| Cyclobutadiene fragments | Terminal | Terminal | Terminal | Internal | Internal | Internal |
| Reactants / kJmol−1 | 156.1804 | 156.1804 | 156.1804 | 156.1804 | 156.1804 | 156.1804 |
| Transition State / kJmol−1 | 241.7481 | 237.7652 | 260.0872 | 275.8218 | 267.9847 | 296.6971 |
| Products / kJmol−1 | 56.3275 | 56.9655 | 0.0026 | 176.7039 | 172.2590 | 203.2740 |
| Activation Energy / kJmol−1 | 85.5676 | 81.5848 | 103.9067 | 119.6414 | 111.8042 | 140.5167 |
| Reaction Energy / kJmol−1 | -99.8530 | -99.2150 | -156.1780 | 20.5235 | 16.0786 | 47.0936 |
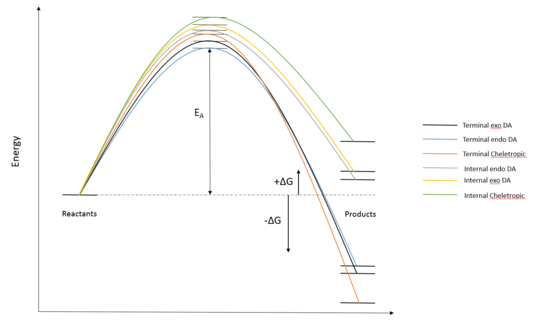
(Don't use curved lines to join stationary points like this. The gradients aren't zero in the diagram as a result Tam10 (talk) 10:59, 23 March 2017 (UTC))
Results of the simulation agrees with experimental data and previous calculation. Products from terminal butadiene fragment reactions are exothermic due aromaticity (visible from IRC). Cheletropic product is the most thermodynamically favoured due to two strong S=O double bond, in addition to the aromaticity. Diels alder reaction on the other hand is more kinetically favoured due to its lower activation energy. The Endo pathway is again more kinetically favourable due to the possible secondary orbital interactions. Products from internal butadiene fragment reactions are endothermic due to ring strain and steric clashes within the products. Activation energy calculation also showed that they are kinetically unfavourable. The endo pathway here is kinetically and thermodynamically most favourable while the Cheletropic pathway is both kinetically and thermodynamically most unfavourable. In conclusion, reactions with internal cyclobutadiene fragments are both kinetically and thermodynamically unfavourable.
(Which experimental data are you comparing to? Tam10 (talk) 10:59, 23 March 2017 (UTC))
Possible Side Reaction

Apart from the above reactions, benzyocyclobutane was accidentally obtained from an ill-fated optimization of the reactant o-Xylene. It should be noted that o-Xylene itself can undergo electrocyclic reaction to form benzocyclobutene (which is aromatic) (Figure 11), reflecting its high thermodynamic instability.
Conclusion
Different types of Diels-Alder and completing reactions were investigated in this experiment. Reactants, products and transition states were optimised to calculate reaction and activation energies. It should be noted that reactant energies were caculated assuming infinite separation, which ignores any interaction energies that molecules would have at close distance, thus not reflecting the true activation energies. Molecular orbitals were also calculated to explain the electron demand of the reactants and visualise the secondary orbitals interactions in transition states. Results showed that sterics of the products and secondary orbital interactions in transition state have to be considered to determine the kinetics and thermodynamics of the reactions.
Reference
Template loop detected: Template:Reflist
- ↑ Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Appendix C: Standard Bond Lengths. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21220/
- ↑ A. Bondi, J. Phys. Chem., 1964, 68, 441–451.