Rep:Mod:ld2416
EX3
BH3
B3LYP/6-31G (d,p)level
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000053 0.001800 YES
RMS Displacement 0.000027 0.001200 YES
Predicted change in Energy=-1.076094D-09
Optimization completed.
-- Stationary point found.
BH3 optimised |
Link to log file: https://wiki.ch.ic.ac.uk/wiki/images/2/25/LM_BH3_FREQ.LOG
Symmetry of Vibrations
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055
Low frequencies --- 1162.9677 1213.1634 1213.1661
Diagonal vibrational polarizability:
0.7180487 0.7179485 1.8415337
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2" E' E'
Frequencies -- 1162.9677 1213.1634 1213.1661
Red. masses -- 1.2531 1.1072 1.1072
Frc consts -- 0.9986 0.9601 0.9601
IR Inten -- 92.5512 14.0538 14.0574
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.16 0.00 0.10 0.00 -0.10 0.00 0.00
2 1 0.00 0.00 -0.57 0.00 0.08 0.00 0.81 0.00 0.00
3 1 0.00 0.00 -0.57 0.39 -0.59 0.00 0.14 -0.39 0.00
4 1 0.00 0.00 -0.57 -0.39 -0.59 0.00 0.14 0.39 0.00
4 5 6
A1' E' E'
Frequencies -- 2582.3761 2715.5558 2715.5570
Red. masses -- 1.0078 1.1273 1.1273
Frc consts -- 3.9598 4.8981 4.8981
IR Inten -- 0.0000 126.3267 126.3171
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.00 0.11 0.00 0.00 0.00 0.11 0.00
2 1 0.00 -0.58 0.00 0.02 0.00 0.00 0.00 -0.81 0.00
3 1 0.50 0.29 0.00 -0.60 -0.36 0.00 -0.36 -0.19 0.00
4 1 -0.50 0.29 0.00 -0.60 0.36 0.00 0.36 -0.19 0.00
Spectrum
Vibration Modes
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2 | yes | out-of-plane bend |
| 1214 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2580 | 126 | E' | Yes | Asymmetric stretch |
| 2713 | 0 | E' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
Ng611 (talk) 23:56, 15 May 2019 (BST) You've gotten two of your entries confused here it seems...
The obtained computational results shows there are 6 vibrational modes, it is expected 3 modes following the 3N-6 rule. The spectrum above only shows 3 peaks, modes 2&3 and modes 5&6 have degenerate energy relative to one another, so only appear as one peak in the spectrum. On the other hand, mode 4 corresponds to a symmetric stretch which does not appear in the spectrum because there's no change in dipole moment involved. That gives two peaks so far, such that the remaining peak is due to non-degenerate out of plane bending.
MO diagram
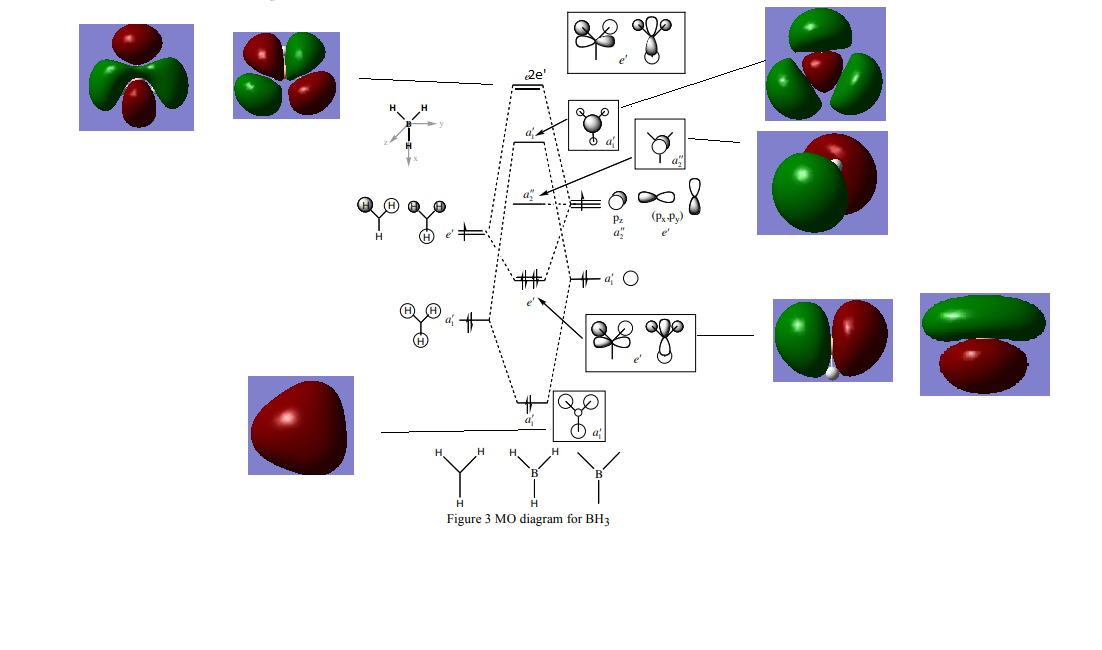 By comparing the quantified MOs obtained in Gaussian to those expected by applying LCAO, it is seen that the MOs come out as expected in terms of relative shape and phases. This is a simple MO diagram because there are only 4 atoms involved in a symmetric arrangement in an 8 electron system.
By comparing the quantified MOs obtained in Gaussian to those expected by applying LCAO, it is seen that the MOs come out as expected in terms of relative shape and phases. This is a simple MO diagram because there are only 4 atoms involved in a symmetric arrangement in an 8 electron system.
Ng611 (talk) 23:57, 15 May 2019 (BST) Can you spot any discrepancies?
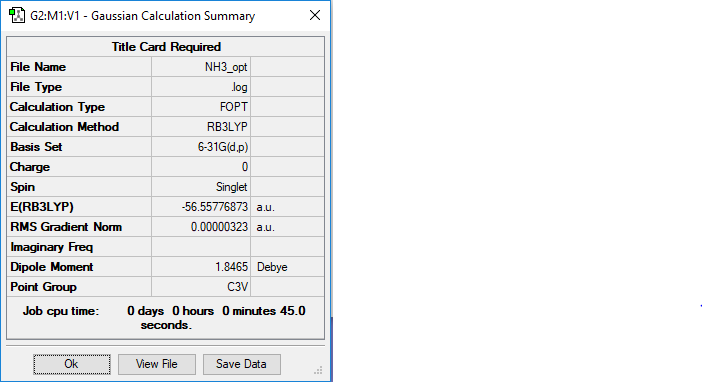
NH3
Item Value Threshold Converged? Maximum Force 0.000092 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000304 0.001800 YES RMS Displacement 0.000101 0.001200 YES
Low frequencies --- -32.4128 -32.3999 -11.4544 -0.0040 0.0076 0.0521 Low frequencies --- 1088.7642 1694.0248 1694.0252
Link to log file: https://wiki.ch.ic.ac.uk/wiki/images/e/e5/NH3_OPT.LOG
NH3 |
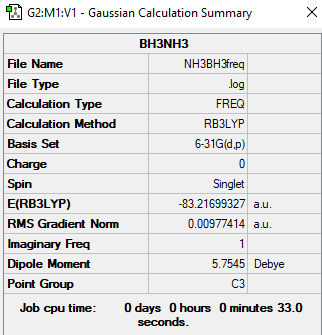
BH3NH3 opt
Item Value Threshold Converged? Maximum Force 0.000114 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000621 0.001800 YES RMS Displacement 0.000355 0.001200 YES
Low frequencies --- -0.0617 -0.0457 -0.0067 21.6783 21.6842 40.5400 Low frequencies --- 266.0169 632.3610 640.1360
Link to log file: https://wiki.ch.ic.ac.uk/wiki/images/6/65/NH3BH3OPT.LOG
NH3BH3 |
Energy Association
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
E(NH3)= -56.5578 a.u.
E(BH3)= -26.6153 a.u.
E(NH3BH3)= -83.2180 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.2180 - (-26.6153-56.5578)
ΔE = -0.0449 a.u. = -135 kJmol-1
The B-N bond is of dative covalent nature, though there's a dipole moment across the bond due to the difference in electronegativity, by comparing to the strength of C-C bond -348 kJmol, C-C is around 2.5 times stronger than the dative covalent B-N bond.
Ng611 (talk) 23:59, 15 May 2019 (BST) Good calculation! But remember... CITE THE SOURCE YOU GOT YOUR BOND ENTHALPIES FROM!
NI3
Item Value Threshold Converged?
Maximum Force 0.000064 0.000450 YES
RMS Force 0.000038 0.000300 YES
Maximum Displacement 0.000488 0.001800 YES
RMS Displacement 0.000278 0.001200 YES
Predicted change in Energy=-6.408178D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -12.7380 -12.7319 -6.2907 -0.0039 0.0188 0.0633 Low frequencies --- 101.0326 101.0333 147.4124
NI3 optimised |
N-I bond length: 2.18362 A
Ng611 (talk) 00:01, 16 May 2019 (BST) Far too much precision here. 3 d.p. is generally as acurate as you can be with your current basis set and XC functional.
Link to log file: https://wiki.ch.ic.ac.uk/wiki/images/2/2b/NI3_GENPP_FEKSYMOPT.LOG
Ionic Liquids
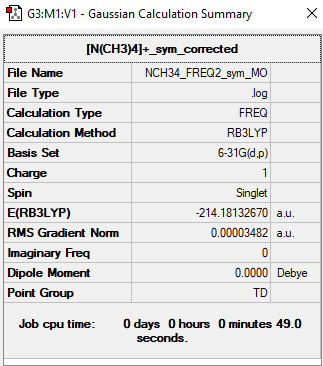
[N(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001086 0.001800 YES RMS Displacement 0.000422 0.001200 YES Predicted change in Energy=-3.639219D-07 Optimization completed.
Low frequencies --- -0.0008 -0.0008 -0.0005 34.4773 34.4773 34.4773 Low frequencies --- 216.6376 316.0116 316.0116
[N(CH3)4]+ |
Link to log file: https://wiki.ch.ic.ac.uk/wiki/images/9/94/NCH34_FREQ2_SYM.LOG
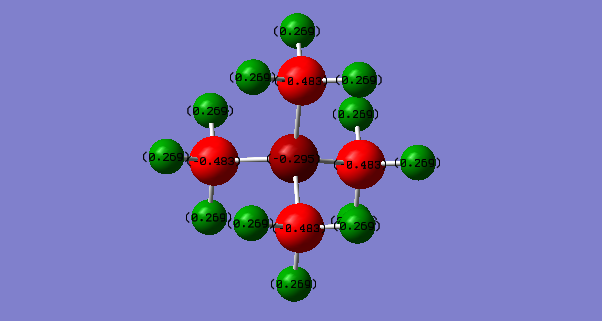
Hydrogens in this system are adjacent to electronegative atoms relative to itself. Electrons are more inclined to be around the nitrogen and less so on the hydrogens. The positive charge is spread across the hydrogens.
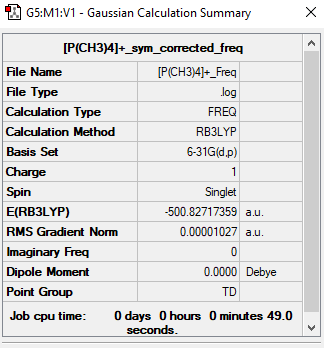
[P(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000050 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000116 0.001800 YES RMS Displacement 0.000086 0.001200 YES Predicted change in Energy=-1.904528D-08
Low frequencies --- -0.0023 -0.0011 -0.0008 50.6326 50.6326 50.6326 Low frequencies --- 187.9314 213.0145 213.0145
[P(CH3)4)]+ |
Freq log file: https://wiki.ch.ic.ac.uk/wiki/images/d/da/P%28CH3%294-%2B_FREQ.LOG
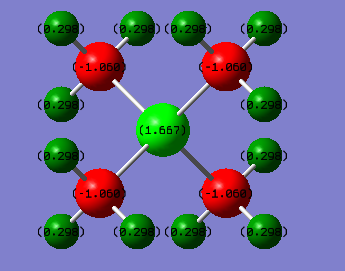
In comparison to [N(CH3)4]+, this ion has its negative charge distributed amongst the methyls because in this system they are the most electronegative species. The positive charge primary sits on the P
Ng611 (talk) 00:06, 16 May 2019 (BST) A side by side comparison (complete with tabulated charges and a more detailed description of differences) would be useful here.
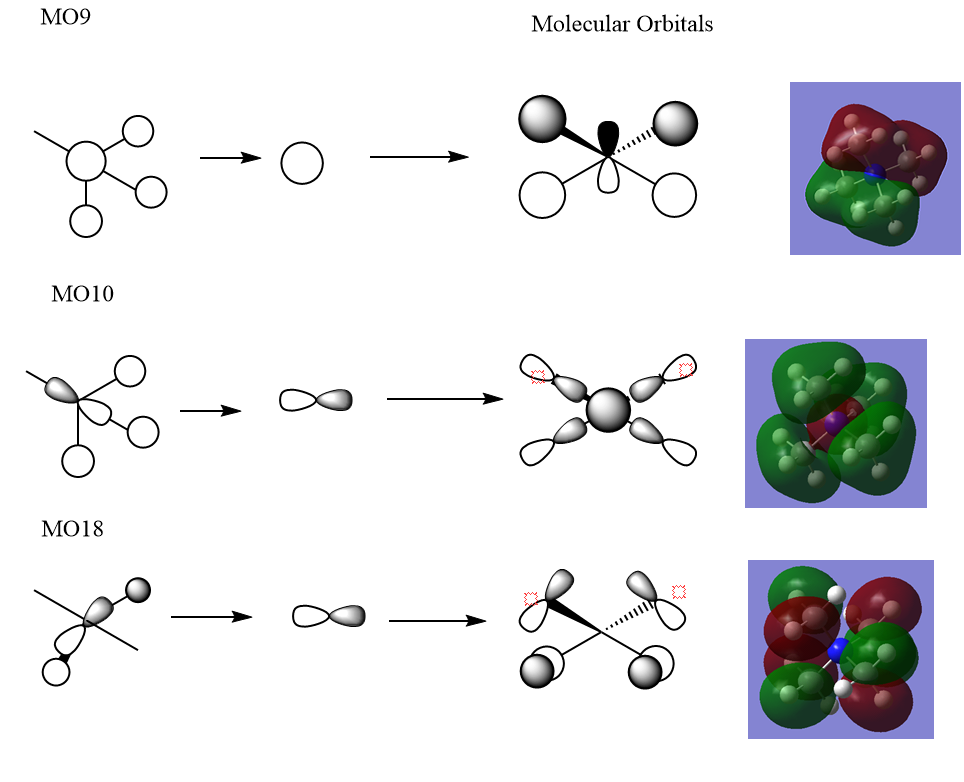
MO 9 is slightly bonding.
MO 10 is bonding
Ng611 (talk) 00:09, 16 May 2019 (BST) Dubious about the FOs for your second LCAO. If you had through bond interactions between your p-like FOs and your central s-orbital, I'd expect a greater amount of electron density. More likely is that the FOs are more like the ones in the first LCAO. Also, labelling some of the key interactions would be useful.