Rep:Mod:kta3817
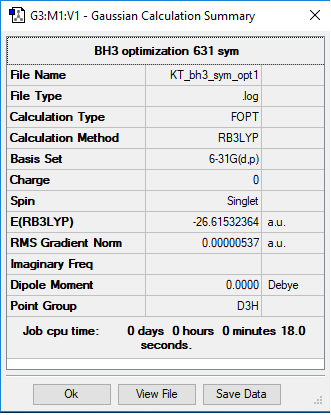
BH3 molecule
B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000043 0.001800 YES
RMS Displacement 0.000028 0.001200 YES
Predicted change in Energy=-6.856714D-10
Optimization completed.
-- Stationary point found.
BH3 frequency .log file: [| BH3.log file]
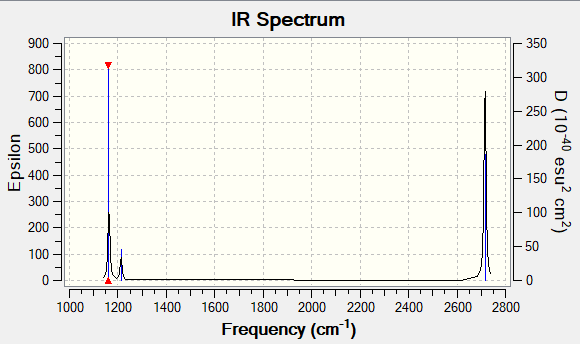
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
Optimized BH3 molecule |
Vibrations
| No | Frequency | Intensity | Bend/stretch | Symmetry | IR active? |
|---|---|---|---|---|---|
| 1 | 1163 | 93 | Bend | A2 | Yes |
| 2 | 1213 | 14 | Bend | E | Yes |
| 3 | 1213 | 14 | Bend | E | Yes |
| 4 | 2582 | 0 | Symmetric stretch | A1 | No |
| 5 | 2716 | 126 | Asymmetric stretch | E | Yes |
| 6 | 2716 | 1126 | Asymmetric stretch | E | Yes |
Only 3 peaks are visible in the IR spectrum. Vibration 4 in the table above is not visible because in order for a bend/stretch to be IR active, the dipole moment of the molecule must change but during symmetric stretch, dipole moment remains the same. Vibrations 2 and 3 have the same frequency and peaks are therefore overlapping, the same happens with vibrations 5 and 6.
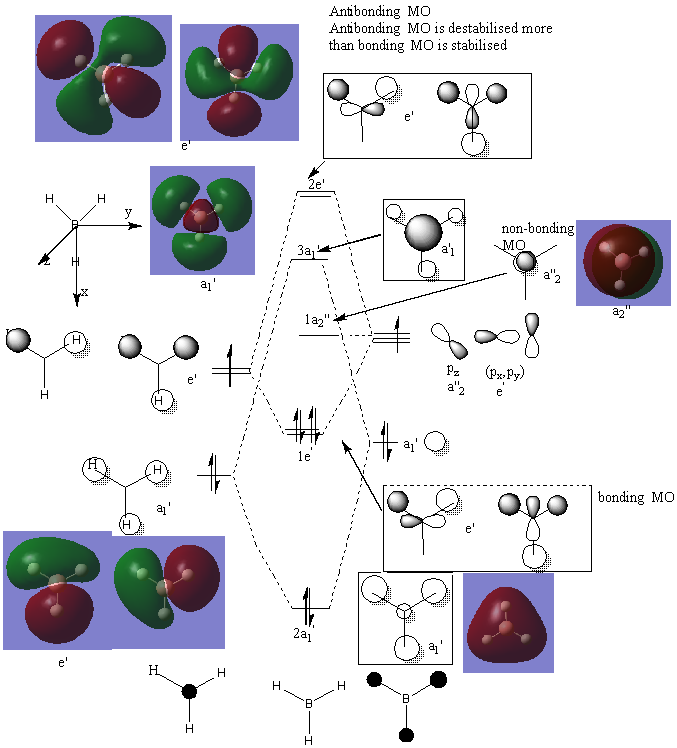
MOs
2a1' LCAO MO is exactly the same as the computed version, however for 1e', the two computed MOs are the same, just in different directions, whereas the LCAO MOs indicate that the MOs are different and that one of them has a B-H bond that is outside both MO phases when this is not actually the case. 1a2 and 3a1' LCAO MOs are exactly the same as their computed versions. 2e' has the same difference that 1e' had: computed MOs are simply different directions but LCAO theory predicts that they have bigger differences and that one of them has a B-H bond that is away from both phases when this is not the case.
Good inclusion of most of the calculated MOs on to the diagram but the labelling could be a little clearer. You have also repeated the same 1e' MO twice, there should have been one without a contribution on the H, and therefore the difference you identified is incorrect. To improve, reconsider the differences between the real and calculated 3a1' MOs for example. Smf115 (talk) 06:48, 30 May 2019 (BST)
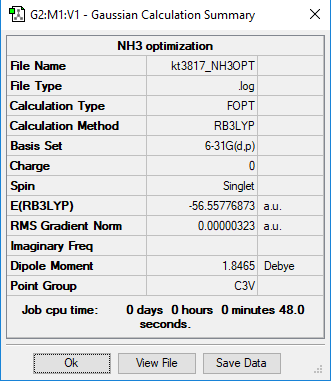
NH3
B3LYP/6-31G(d,p)
NH3 frequency .log file: Media:KT3817_NH3OPT_FREQ.LOG
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.844611D-11
Optimization completed.
-- Stationary point found.
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Optimized NH3 molecule |
NH3BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000114 0.000450 YES
RMS Force 0.000064 0.000300 YES
Maximum Displacement 0.000617 0.001800 YES
RMS Displacement 0.000362 0.001200 YES
Predicted change in Energy=-1.787894D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -827.7026 -33.9159 -19.3033 -3.1260 0.0002 0.0010 Low frequencies --- 0.0011 716.1684 743.0238
NH3BH3 frequency .log file: [| NH3BH3 .log file]
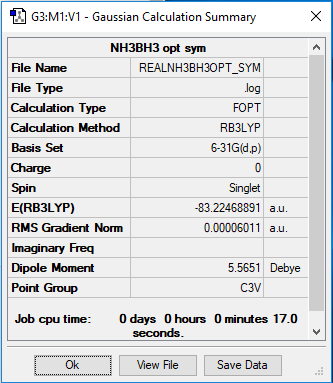
Optimized NH3BH3 molecule |
E(BH3)= -26.61532 hartree
E(NH3)= -56.55777 hartree
E(NH3BH3)= -83.22469 hartree
ΔE = -83.22469 + 56.5577 + 26.61532 = -0.05160 hartree = -136 kJ/mol
Is the B-N bond weak, medium or strong?
Based on the calculation, I would expect B-N bond to be medium as it is the only forming bond in the reaction and the reaction is exothermic but not as exothermic as the reactions where very strong bonds are formed. For example, formation energy for C=O is -393.5 kJ/mol.
Correct calculation, clearly reported energies for the molecules and good consideration of the accuracy of the final value. Note that all bond forming is exothermic though and consider whether C=O is a good comparative bond strength to be using? Also always reference literature values! Smf115 (talk) 06:52, 30 May 2019 (BST)
NI3
B3LYP/GEN
Item Value Threshold Converged?
Maximum Force 0.000139 0.000450 YES
RMS Force 0.000090 0.000300 YES
Maximum Displacement 0.001129 0.001800 YES
RMS Displacement 0.000796 0.001200 YES
Predicted change in Energy=-1.675756D-07
Optimization completed.
-- Stationary point found.
NI3 frequency .log file: Media:ktni3_freq.log
Low frequencies --- -12.7179 -12.7118 -6.4125 -0.0039 0.0189 0.0621 Low frequencies --- 101.0754 101.0761 147.4556
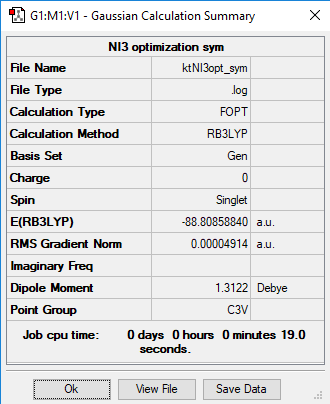
Optimized NI3 molecule |
Optimized N-I distance is 2.184 Å.
Ionic liquids
N(CH3)4+
B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000023 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.001012 0.001800 YES
RMS Displacement 0.000229 0.001200 YES
Predicted change in Energy=-1.320388D-08
Optimization completed.
-- Stationary point found.
N(CH4)+ frequency .log file: Media:kt3817_n(ch3)4+opt1_freq.log
Low frequencies --- -0.0014 -0.0012 -0.0012 34.9548 34.9548 34.9548 Low frequencies --- 217.5087 316.5877 316.5877
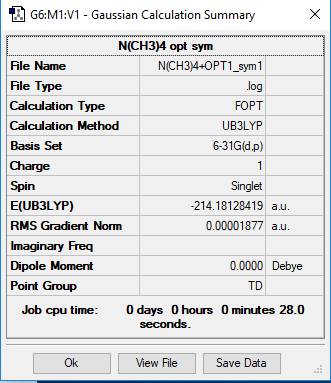
Optimized NH3 molecule |
P(CH3)4+
B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000138 0.000450 YES
RMS Force 0.000032 0.000300 YES
Maximum Displacement 0.000792 0.001800 YES
RMS Displacement 0.000286 0.001200 YES
Predicted change in Energy=-1.621425D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -0.0034 -0.0032 -0.0023 51.2606 51.2606 51.2606 Low frequencies --- 186.5808 211.3947 211.3947
P(CH4)+ frequency .log file: Media:Kt3817_P(CH3)4OPT_SYM1.LOG
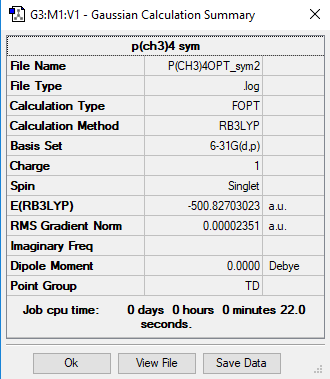
Optimized NH3 molecule |
Charge distribution
| N(CH3)4+ | P(CH3)4+ |
|---|---|
 |
|
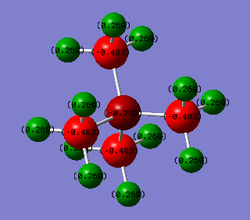
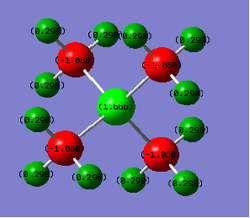
In the N(CH4)+ image above, N has a charge of -0.295, C has a charge of -0.483 and H has a charge of 0.269. In the P(CH4)+ image above, P has a charge of 1.666, C has a charge of -1.050 and H has a charge of 0.298.
In N(CH3)4+, central atom N has a negative charge, however in P(CH3)4+, central atom P has a positive charge. In both molecules, C has the largest negative charge of all the atoms and H atoms have positive charge. In P(CH3)4+, P has the largest positive charge located on it, whereas H atoms are the only ones in N(CH3)4+ to have positive charge. In N(CH3)4+, atoms with a negative charge are bonded (N and C) whereas in P(CH3)4+, the charges are alternating as P has positive charge, C that is bonded with it has negative charge and H that is bonded with C has positive charge.
In the classical N(R)4+ charge distribution, N is depicted to have a positive charge since N in ammonium ion has 1 less free electron pair than the neutral N atom. Computed charge distribution, however, shows that N has negative charge and the only atoms carrying positive charge are H atoms.
Correct NBO charges calculated, however, you should have used the same colour range to display the charges over both of the molecules. You have only described the differences across the two ILs and haven't attempted to analyse or explain the distributions. To improve you should consider how the charges arise from electronegativities and symmetries, for example, and your explanation of how the formal charge arises is also not correct (think about Lewis structures and formal electron counting). Smf115 (talk) 14:37, 30 May 2019 (BST)
MOs
| No | MO | LCAO MO |
|---|---|---|
| 9 | 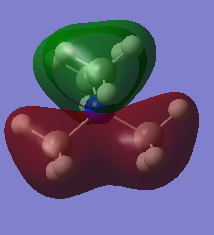 |
|
MO 9 pictured above is bonding and occupied.
| No | MO | LCAO MO |
|---|---|---|
| 10 | 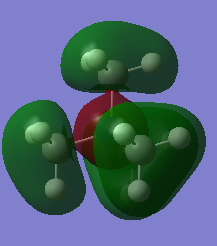 |
|
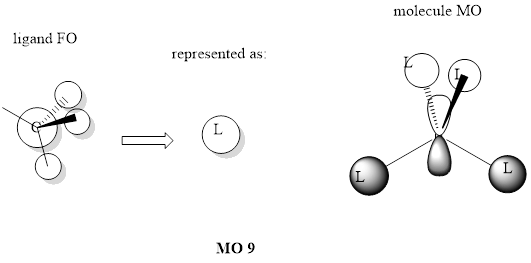
MO 10 pictured above is antibonding if the molecule is pictured as NL4+ but is bonding for methyl group fragment orbitals. Methyl group FOs are in the opposite phase in the molecule compared to the figure which shows how they are formed. MO is occupied.
| No | MO | LCAO MO |
|---|---|---|
| 19 | 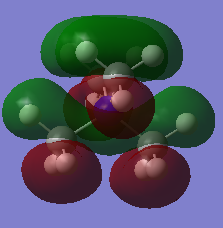 |
|
MO 19 pictured above is bonding overall and occupied.
Good construction of the FOs and corresponding LCAOs and clear presentation! To improve it would have been nice to see some evaluation of the main interactions in the LCAO to show where the overall MO character arises from. Smf115 (talk) 14:42, 30 May 2019 (BST)
Overall a good report! Smf115 (talk) 14:42, 30 May 2019 (BST)