Rep:Mod:kl88123
Many aspects of organic structure and reactivity can now be accurately predicted simply by using a computer, and such modelling is not limited to rationalising the results of reactions, it can actually be used to predict new types of reaction, and various information of molecules such as NMR spectra and optical rotation can also be predicted. Various modelling experiments will be investigated in this experiments and the diversity to real experiments will be illustrated.
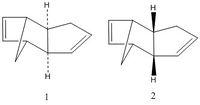
The dimerisation of cyclopentadiene Cyclopentadiene can dimerise to give either "1" the exo product or "2" endo product. It is proven that the endo product is the dominated product and this exercise will examine whether the reaction is under kinetic or thermodynamic control by optimising the geometry of the two isomers. Calculation was performed in ChemBio3D and the MM2 force field is applied to optimise the geometry of the molecule to give the conformation that has the lowest energy. The total energy and each energy component of the endo and exo porduct is summarised in the table below.
| Type of Energy | Exo(kcal/mol) | Endo(kcal/mol) |
| Stretch | 1.2851 | 1.2509 |
| Bend | 20.5805 | 20.8477 |
| Stretch Bend | -0.8381 | -0.8358 |
| Torsion | 7.6555 | 9.5109 |
| Non 1,4 VDW | -1.4174 | -1.5439 |
| 1,4 VDW | 4.2333 | 4.3200 |
| Dipole/Dipole | 0.3775 | 0.4476 |
| Total Energy | 31.8764 | 33.9975 |
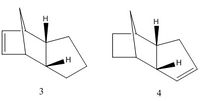
The Hydrogenation of the Cyclopentadiene Dimer
This reaction is regioselective as there are two different alkene functional groups within the molecule, the tetrahydro specie will only form after prelonged hydrogenation. Therefore, either product 3 or product 4 is initially formed. This exercise will determine which of the product is thermodynamically most stable, ie the one with a lower energy. Again the MM2 force field is applied to calculate the energy of the two compounds and the results are summarized in the table below.
| Type of Energy | Product 3 | Product 4 |
| Stretch | 1.2348 | 1.0965 |
| Bend | 18.9382 | 14.5243 |
| Stretch Bend | -0.7609 | -0.5494 |
| Torsion | 12.1240 | 12.4974 |
| Non 1,4 VDW | -1.5015 | -1.0700 |
| 1,4 VDW | 5.7289 | 4.5126 |
| Dipole/Dipole | 0.1631 | 0.1406 |
| Total Energy | 35.9266 | 31.1520 |
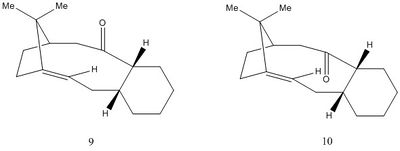
Atropisomerism in an intermediate related to the synthesis of Taxol
Isomer 9 and 10 cannot ring flip due to the strong C=C bond adjacent to bridge head. Therefore in synthsising taxol, the carbonyl group either points up or down. The conformation with the carbonyl pointing up has a energy of 70.5 kcal/mol whereas the conformation with the carbonyl pointing downwards has a energy of 74.4 kcal/mol. When we compare the bending energy of the two comformations, isomer 10 has a much lower value. This suggests that relevant atoms are arranged at a favorable angle, allowing orbitals to overlap smoothly. The 2 hydrogen atoms are arranged anti peri-planar with respect to the carbonyl group. This is the dominating interaction, therefore giving a lower energy conformation. Isomer 9 do not enjoy this interaction, as a result it has a higher energy. This reaction is thermodynamically control so the most stable product will form ie isomer 10.[1]
The derivative of 10
Isomer 18(The derivative of 10) is shown on the right. We can use Avogadro to compute the 1H and 13C NMR with an appropriate solvent (Benzene) and compare with the ones in literature.

The results are shown below
| Atom Number | Degeneracy | Chemical Shift PPM | Chemical Shift from literature [2] |
| 24 | 1 | 6.32 | 5.21 |
| 52 | 3 | 3.31 | 2.70-3.00 |
| 51 | 3 | 3.30 | 2.70-3.00 |
| 50 | 3 | 3.27 | 2.70-3.00 |
| 29 | 1 | 3.15 | 2.70-3.00 |
| 53 | 1 | 3.09 | 2.70-3.00 |
| 26 | 1 | 2.98 | 2.70-3.00 |
| 25 | 2 | 2.87 | 2.70-3.00 |
| 32 | 2 | 2.84 | 2.70-3.00 |
| 20 | 2 | 2.71 | 2.70-3.00 |
| 36 | 2 | 2.67 | 2.70-2.35 |
| 22 | 1 | 2.51 | 2.70-2.35 |
| 27 | 1 | 2.32 | 2.70-2.35 |
| 35 | 1 | 2.24 | 2.20-1.70 |
| 41 | 1 | 2.14 | 2.20-1.70 |
| 43 | 1 | 1.95 | 2.20-1.70 |
| 28 | 3 | 1.89 | 2.20-1.70 |
| 23 | 3 | 1.89 | 2.20-1.70 |
| 21 | 3 | 1.88 | 2.20-1.70 |
| 34 | 1 | 1.66 | 2.20-1.70 |
| 33 | 3 | 1.60 | 1.58 |
| 37 | 3 | 1.58 | 1.50 |
| 30 | 3 | 1.57 | 1.50 |
| 42 | 1 | 1.46 | 1.50 |
| 45 | 1 | 1.30 | 1.50-1.20 |
| 40 | 4 | 1.07 | 1.50-1.20 |
| 44 | 4 | 1.05 | 1.10 |
| 38 | 4 | 1.06 | 1.45 |
| 31 | 4 | 1.00 | 1.07 |
| 39 | 1 | 0.68 | 1.03 |
| Atom Number | Degeneracy | Chemical Shift PPM | Chemical Shift from literature[2] |
| 9 | 1 | 215.1 | 212.1 |
| 3 | 1 | 143.9 | 148.8 |
| 4 | 1 | 124.1 | 120.9 |
| 15 | 1 | 88.0 | 74.6 |
| 6 | 1 | 59.0 | 60.5 |
| 11 | 1 | 55.8 | 51.3 |
| 7 | 1 | 52.6 | 50.9 |
| 16 | 1 | 49.9 | 43.8 |
| 13 | 1 | 49.9 | 43.8 |
| 48 | 1 | 46.0 | 40.8 |
| 49 | 1 | 42.1 | 38.7 |
| 8 | 1 | 41.8 | 38.7 |
| 12 | 1 | 36.4 | 36.8 |
| 2 | 1 | 34.2 | 35.5 |
| 5 | 1 | 29.2 | 30.8 |
| 17 | 1 | 28.0 | 30.0 |
| 19 | 2 | 26.4 | 25.6 |
| 1 | 2 | 26.3 | 25.4 |
| 18 | 1 | 25.7 | 22.1 |
| 14 | 3 | 20.8 | 21.4 |
The results generally agrees well with literature. However, there are some peaks that have a notable difference. From the 13C NMR spectra, the shift from carbon 15 has the the biggest deviation from experimental results, with a difference of 13.4 ppm. This carbon is bounded to two sulfur atoms so spin-obit coupling errors are very likely to be present and as a result, there will be a deviation to literature data. Nonetheless, most other resonances agrees well with literature values.
The epoxidation of alkenes
The two alkenes that I chose to investigate is beta methyl styrene and 1,2-dihydronapthaline. The general reaction scheme is shown below:
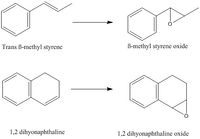
This reaction is both regio and stereo selective, for the first reaction shown, the reaction can give cis or trans epoxide.
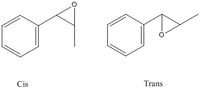
The product will also form two chiral centers, giving four enantiomers namely(R,S),(S,R),(R,R) and (S,S) as shown on the right.

By using the MMFF94s force field, the configuration of the trans and cis are optimized and a total energy is calculated.
It is found that the trans form(23.17 kcal/mol) has a lower energy than the cis form (23.91 kcal/mol). Therefore we should focus on the trans form. The NMR is calculated and the results are shown below.

| Atom Number | Degeneracy | Chemical Shift PPM | Chemical Shift from literature[3] |
| 15 | 3 | 7.50 | 7.25-7.37 |
| 12 | 3 | 7.49 | 7.25-7.37 |
| 14 | 3 | 7.48 | 7.25-7.37 |
| 13 | 1 | 7.42 | 7.25-7.37 |
| 11 | 1 | 7.37 | 7.25-7.37 |
| 16 | 1 | 3.41 | 3.50 |
| 17 | 1 | 2.79 | 3.01-3.07 |
| 20 | 1 | 1.68 | 3.01-3.07 |
| 19 | 1 | 1.59 | 1.45 |
| 18 | 1 | 0.72 | 1.45 |

| Atom Number | Degeneracy | Chemical Shift PPM | Chemical Shift from literature[3] |
| 6 | 1 | 135.0 | 138.0 |
| 2 | 1 | 124.1 | 128.7 |
| 4 | 1 | 123.3 | 125.8 |
| 5 | 1 | 122.8 | 125.8 |
| 3 | 1 | 122.7 | 125.8 |
| 1 | 1 | 118.5 | 125.8 |
| 8 | 1 | 62.3 | 59.9 |
| 7 | 1 | 60.6 | 59.4 |
| 9 | 1 | 18.8 | 18.3 |
The results agrees very well with the literature data as there are no heavy atoms in the molecules nor any double bonds connected to hetero atoms.
The 1H and 13C NMR of cis beta methyl stylene was also generated to compare with the trans product. The results are shown below.

| Atom Number | Degeneracy | Chemical Shift PPM |
| 15 | 3 | 7.55 |
| 14 | 3 | 7.52 |
| 12 | 3 | 7.52 |
| 13 | 1 | 7.46 |
| 11 | 1 | 7.39 |
| 16 | 1 | 4.00 |
| 17 | 1 | 3.31 |
| 18 | 1 | 1.45 |
| 19 | 1 | 0.80 |
| 20 | 1 | 0.65 |
In general the chemical shifts are lower for each corresponding resonance to that of the trans product.

| Atom Number | Degeneracy | Chemical Shift PPM |
| 6 | 1 | 132.8 |
| 4 | 1 | 123.9 |
| 2 | 1 | 123.1 |
| 5 | 1 | 122.7 |
| 3 | 1 | 122.4 |
| 1 | 1 | 121.8 |
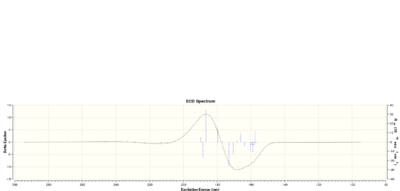
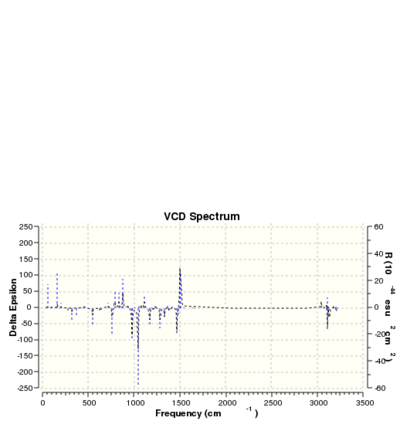
We can also work out the chiroptical property of the epoxide. The optical rotation of a particular enantiomer is calculated and compared with literature. I have chosen to calculate the epoxide group pointing downwards as before. The optical rotation value I calculated is 5890.0 A = 183.12 deg (Lit -116 deg)[5]. We can also use ECD and VCD to characterise the exact absolute configuration of the alkene. The results are shown below.
For the epoxidation of 1,2 dihydronapthaline, only the trans product will be produced since the reactant is a cyclic six membered ring and the molecule is symmetrical ie does not matter whether epoxide approach from above or below the plane. Like the previous reaction, this reaction would still form a racemic of enantiomeric species .

| Atom Number | Degeneracy | Chemical Shift PPM | Chemical Shift from literature[4] |
| 15 | 3 | 7.51 | 7.09-7.26 |
| 13 | 3 | 7.42 | 7.09-7.26 |
| 14 | 3 | 7.37 | 7.09-7.26 |
| 12 | 1 | 7.21 | 6.55-6.57 |
| 16 | 1 | 3.62 | 3.04-3.06 |
| 17 | 2 | 3.58 | 3.04-3.06 |
| 20 | 1 | 2.89 | 3.04-3.06 |
| 21 | 1 | 2.28 | 3.04-3.06 |
| 18 | 1 | 2.21 | 3.04-3.06 |
| 19 | 1 | 1.58 | 3.04-3.06 |
| Atom Number | Degeneracy | Chemical Shift PPM |
| 6 | 1 | 133.8 |
| 5 | 1 | 130.4 |
| 4 | 1 | 125.5 |
| 1 | 1 | 124.2 |
| 2 | 1 | 124.6 |
| 3 | 1 | 122.6 |
| 8 | 1 | 56.5 |
| 7 | 1 | 54.8 |
| 10 | 1 | 28.0 |
| 9 | 1 | 25.0 |
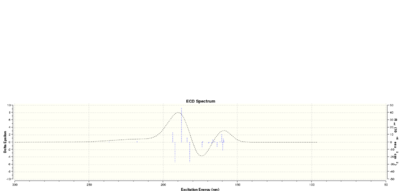
Like in the previous case, we can also work out the chiroptical property of the epoxide via HPC. The optical rotation of a particular enantiomer is calculated and compared with literature. I have chosen to calculate the epoxide group pointing downwards as before. The optical rotation value I calculated is 5890.0 = -103.16 deg(Lit -116 deg[6]. We can also use ECD and VCD to characterise the exact absolute configuration of the alkene. The results are shown below.
Pre-active form of the Shi catlayst
The pre-active form of the Shi catlayst is shown below. The bond distant between all the O-C-O substructure are shown below. There are three O-C-O substructure but only two of them can be classed as anomeric centers. The carbon next to "1.408" is connected to two oxygen atoms but this is not an anomeric center because one of the oxygen is in a 6 membered ring and the other is in 5 membered ring. As a result, orbitals are of the wrong hybridization to overlap with each other and also they are not aligned in a anti pBold texteriplanar fashion so the anomeric effect is much less likely to occur.
Pre-active form of the Jacobsen catlayst
The pre-active form of the Jacobsen catlayst is shown below. The distant between the two tertiary butyl group is very similar to 2 x VDW of C, Therefore, this is a very good distant to prevent itself reacting with the alkene and as a result, it is not the active form for epoxidation.
References
1.Evaluation and Prediction of the Stability of Bridgehead Olefins, Wilhelm F. Maier* and Paul von RaguC Schleye, J. Am. Chem. SOC. 1981, 103, 1891-1900
2. [ 3.31 Sigmatropy within 1 -Vinyl-2-alkenyl-7,7-dimethyl-exo-norbornan-2-2-, Leo A. Paquette,* Neil A. Pegg, Dana Toops,’ George D. Maynard, and Robin D. Roger J. Am. Chem. SOC. 1990, 112, 211-283
3. Olefin Epoxidations Using the Dicyclohexylcarbodlimide-H 20 2 System, Robert W. Murray* and Kaliappan lyanarJ. Org. Chem., 1998, 63(5), 1730-1731, DOI:10.1021/jo9719160
4.Initial reaction in the oxidation of 1,2-dihydronaphthaline by Sphingomonas yanoikuyae strains, S L Eaton, S M Resnick and D T Gibson Appl. Environ.microbiol. 1996, 62(12):4388
5. Boyd, Derek R.; Sharma, Narain D.; Agarwal, Rajiv; Kerley, Nuala A.; McMordie, R. Austin S.; et al. Journal of the Chemical Society, Chemical Communications, 1994 , # 14 p. 1693 - 1694].
6. Fourneau; Benoit Bulletin de la Societe Chimique de France, 1945 , vol. <5> 12, p. 985,988