Rep:Mod:kc8
Module 1
Modelling using Molecular Mechanics
Molecular mechanics (MM) provides a method of investigating certain aspects of a molecule such as the optimised geometry for a particular conformation, the relative stability of the molecule (given by the so called 'Total Energy') and the contribution of steric effects, van der Waals interactions etc to the overall stability of the molecular system without explicitly solving the Schrodinger equation. This means that the method can be run in a short amount of time, although the results can only be used in a relatively qualitative manner. Molecular mechanics assumes that the energy of molecular can be approximated by the contribution of five independent terms:
- The sum of the diatomic bond stretches (approximated with the Hookes law potential)
- The sum of the triatomic bond angle deformations (bending and scissoring, approximated as above)
- The sum of the tetra-atomic bond torsions (cosine dependence on dihedral angle)
- The sum of the Van der Waals attractions and repulsions (approximated using a simple Lennard-Jones 6-12 potential)
- The sum of the electrostatic attractions between bond dipoles (hydrogen bonding, Coulomb interaction)
As each of the functions are independent they are mathematically easy to solve and can be computed quickly. The modelling method is limited in that it depends on known parameters such as force constants for Hookes law, bond dipole moments, etc. Therefore the method begins to fall apart when modelling non-classical molecular systems or aromatic systems or molecules which contain certain types of bonds for which the force constants are not known.
In this experiment, the Allinger MM2 molecular mechanics module will be used as implemented in the ChemBio3D program, and occassionally the MMFF94 module.
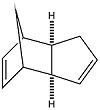
The Hydrogenation of Cyclopentadiene Dimer
At room temperature, cyclopentadiene dimerises to form the endo dimer rather than the exo dimer. The reasons for the stereospecificity of this reaction can be investigated by comparing the relative energies of the two products through Molecular Mechanics. Using the MM2 force field, the geometries of the two isomers their relative stabilities was computed.
 |

|
| Exo Dimer | Endo Dimer |
| Isomer | 3D representation | Total Energy/ kcal mol-1 |
|---|---|---|
| Exo Dimer | 31.77
| |
| Endo Dimer | 33.92
|
The computational results show that the exo dimer would be the thermodynamic product and is 2.15 kcal mol-1more stable than the endo dimer. However since we know that the endo dimer is formed, the reaction must be kinetically controlled and the transition state for the formation of the endo dimer must be lower in energy than that of the exo dimer.
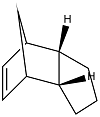
The partial hydrogenation of the endo dimer can occur at two different places to give two different products A and B shown below. The relative contributions from the stretching, bending, stretch-bend, torsion, van der Waals and hydrogen bonding energy terms are shown together with the combined total energy.
 |

|
| Product A | Product B |
| Isomer | 3D representation | Stretching/ kcal mol-1 | Bending/ kcal mol-1 | Stretch-bend/ kcal mol-1 | Torsion/ kcal mol-1 | van der Waals/ kcal mol-1 | Hydrogen bonding/ kcal mol-1 | Total Energy/ kcal mol-1 |
|---|---|---|---|---|---|---|---|---|
| Product A | 1.28 |
19.80 |
-0.84 |
10.87 |
4.42 |
0.16 |
35.70
| |
| Product B | 1.10 |
14.55 |
-0.55 |
12.50 |
3.41 |
0.14 |
31.16
|
The data shows that product B is 4.54 kcal mol-1 more stable than product A. This is mainly due to the particularly high bending strain term in product A. The cause of this strain may be due to the repulsions between the alkene and the bending of the bridge.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
The reaction of an optically active derivative of prolinol with methyl magnesium iodide proceeds as shown in the reaction scheme below. The stereochemistry of the product is absolute as shown in the product.
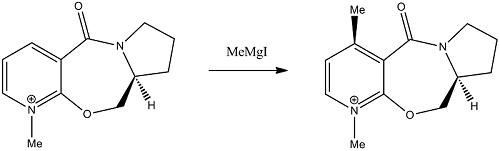
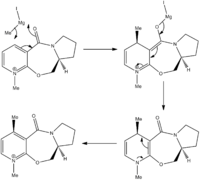
The regio- and stereoselectivity of the reaction can be rationalised through the involvement of the carbonyl of the amide group. In the first step of the mechanism, the oxygen of the amide group coordinates to the magnesium of the Grignard reagent and acts as an anchor.[1] [2] The delivery of the methyl group is therefore directed to the 4-position of the pyridinium ring through the formation of an intermediate containing a six-membered ring. This explains the regioselectivity of the reaction. In order to explain the stereoselectivity we must first consider the geometry of the prolinol derivative reactant.
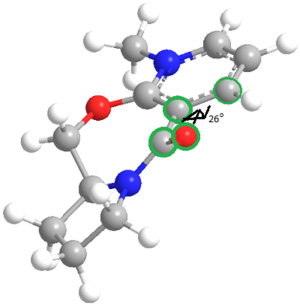
The geometry of the prolinol derivative was optimised using MM2. This model was used to calculate the dihedral angle O-C-C-C, shown visually in the image on the right. The green highlighted atoms are the four atoms for which the dihedral angle was calculated. However due to a bug in the ChemBio3D program which affects molecules containing an N+ atom, a second optimisation using the MOPAC PM6 molecular orbital method was used in order to give a more accurate geometry.
| Method | Total Energy/kcal mol1 | Dihedral Angle/o |
|---|---|---|
| MM2 | 43.11 |
10
|
| MMFF94 | 56.46 |
15
|
| MOPAC | Heat of Formation = 93.89 kcal mol-1 |
26
|
A 3D rotatable model of the MOPAC optimised geometry can be seen by clicking the button below.
The dihedral angle found when optimised using the MM2 method and using the MOPAC method differed significantly demonstrating the inaccuracy in of running the MM2 method. The MMFF94 method resulted in a geometry of higher energy although the dihedral angle was closer to the MOPAC result. The 3D model shows the significant distortion of the carbonyl group 'above' the pyridinium ring, and due to the nature of the mechanism explained before, this results in the addition of the methyl group 'above' the plane of the pyridine ring, explaining the stereoselective result of the reaction. The bending of the carbonyl out of the plane of the ring is thought to result from a repulsive interaction with the lone pair on the nitrogen, pointing downwards away from the ring.
This stereoselectivity is also seen in another reaction involving similar functional groups but reacting with aniline.
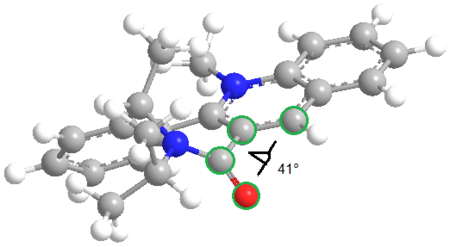
The amide O-C-C-C dihedral angle was once again measured using the three different models.
| Method | Total Energy/kcal mol1 | Dihedral Angle/o |
|---|---|---|
| MM2 | 62.60 |
-21
|
| MMFF94 | 98.38 |
-36
|
| MOPAC | Heat of Formation = 155.46 kcal mol-1 |
-41
|
The regioselectivity of the reaction is facile since there is only the 4-position on the pyridinium ring is available for attack. The stereselectivity occurs predominantly due to Coulombic repulsions between the lone pair on the amide oxygen and the lone pair on the incoming aniline. As the carbonyl is pointing 'down' with respect to the planar pyridinium ring, the aniline nitrogen must therefore attack from above the plane of the ring to minimise repulsions. This results in the stereoselectivity for the aniline to be in the 'up' position with respect to the pyridinium ring.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
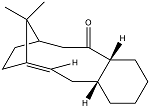
Part of the total synthesis of Taxol involves the intermediates shown below. The carbonyl group in the molecule can either point 'up' or 'down' and upon standing it has been observed that the equilibrium of atropisomers shifts to a single atropisomer. Atropisomerism occurs due to restricted rotation around a single bond usually as a cause of large steric hindrance. This occurs in the following two intermediates because the carbonyl group is unable to switch from the 'up' and the 'down' position by rotation inwards within the ring, due to steric repulsions. Therefore in order for interconversion to occur, the carbonyl group must rotate outwards, which also exerts some strain on the bonds within the ring. Therefore there is a restricted rotation around the single bonds either side of the carbonyl group.
 |
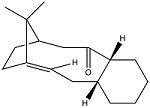
|
The relative energies of each atropisomer were computed using the MM2 and MMFF94 methods in order to determine which of the two was more stable.
| Isomer | Stretching/ kcal mol-1 | Bending/ kcal mol-1 | Stretch-bend/ kcal mol-1 | Torsion/ kcal mol-1 | van der Waals/ kcal mol-1 | Hydrogen bonding/ kcal mol-1 | MM2 Total Energy/ kcal mol-1 | MMFF94 Total Energy/ kcal mol-1 |
|---|---|---|---|---|---|---|---|---|
| A | 2.65 |
15.82 |
0.38 |
18.26 |
11.62 |
0.15 |
48.88 |
70.54
|
| B | 2.55 |
10.68 |
0.32 |
19.72 |
11.20 |
-0.18 |
44.28 |
60.55
|
The data shows that Atropisomer B is more stable than Atropisomer A by 4.60 kcal mol-1. The largest contribution to this increase in energy in Atropisomer A can be attributed to the bending energy from the table.
The relative energies calculated using the MMFF94 method are considerably higher than those calculated using the MM2 method. However the data still shows that Atropisomer B is more stable than Atropisomer A, although by a larger margin of 9.99 kcal mol-1.
Hyperstable Alkenes
The hydrogenation of this alkene to form the alkane is known to occur slowly. This is because these intermediates can be classified as hyperstable alkenes.[3] In general, the Strain Energy (Est) of a cyclic alkene is higher than that of the corresponding alkane. This is because cyclic alkenes have a positive Olefin Strain Energy (OSE). However, cyclic alkenes have been found in which the OSE is in fact negative, although still slightly higher than that of the corresponding alkane, and these alkenes are known as hyperstable alkenes. The reason for the lower OSE is due to an increase in the number of vicinal and transannular hydrogen van der Waals interactions which stabilise the alkene. Hyperstable alkenes are known to be particularly difficult to hydrogenate, even using catalysts, and this would explain the slow reaction of these intermediates with hydrogen.
Modelling using Semi-empirical Molecular Orbital Theory
The advantages and disadvantages of using molecular mechanics to model molecular systems have been demonstrated in the analyses above. The advantages of taking into account the electronic aspects of reactivity will be shown in the following exercises in which we can predict more accurately the reactivity of particular functional groups in a molecule and spectroscopic properties of the system.
Regioselective Addition of Dichlorocarbene
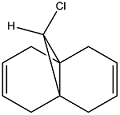
The reactivity of Compound A with electrophilic reagents such as dichlorocarbene or peracid can be predicted by using the MOPAC/PM6 method. This method calculates the energies of the orbitals and can display them graphically allowing us to determine which of the two alkene groups is more reactive to electrophilic attack.
In the first step the molecule was optimised using the MM2 method in order to give the geometry to a reasonable level of accuracy. This was then optimised using the MOPAC/PM6 method with a heat of formation of 19.74 kcal mol-1.
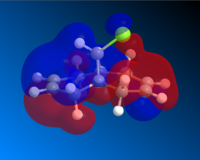
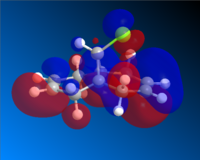
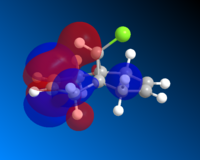
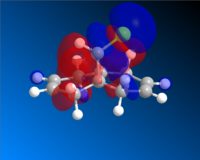
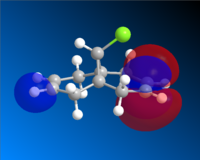
Having completed the MOPAC/PM6 method, the molecular orbitals for Compound A could be seen visually. The HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2 are shown below.
 |
 |
 |
 |
|
HOMO-1 |
HOMO |
LUMO |
LUMO+1 |
LUMO+2
|
Looking first at the HOMO, it can be seen that there is a greater degree of electron density at the endo alkene (on the same side as the chlorine atom) than at the exo alkene. As the incoming dichlorocarbene acts as an electrophile, the relative reactivity of each of two alkenes in the system would be related to the electron density in the π-bond. Therefore we can say that the endo alkene is more reactive than the exo alkene.
The geometry of the molecule was further optimised using B3LYP/6-31G(d,p) ran on the SCAN. This also produced a predicted IR absorption spectrum by calculating the bond strengths. The absorption frequencies for the C=C and C-Cl bonds in Compound A and also the exo-hydrogenated product were obtained and compared in order to investigate the effect of the Cl-C bond on the vibrational frequencies of the molecule. For further investigation, the exo CH2 was also replaced with several different substituents to observe the effect of the substituents on the vibrational frequencies of the molecule.
| Compound | endo C=C | exo C=C | C-Cl |
|---|---|---|---|
| A | 1757.36 (3.94) |
1737.09 (4.20) |
770.90 (25.12)
|
| dihydro | 1758.05 (4.34) |
N/A |
774.94 (20.02)
|
| C-OH | 1757.76 (37.33) |
1753.00 (59.40) |
765.28 (6.67)
|
| C-CN | 1756.54 (4.82) |
1706.31 (11.43) |
765.79 (8.07)
|
| C-BH2 | 1756.56 (4.53) |
1657.22 (131.94) |
759.04 (3.83)
|
| C-SiH3 | 1756.25 (5.51) |
1690.34 (19.04) |
763.83 (17.43)
|
Surprisingly, the absorption frequency for the endo C=C bond is higher than that for the exo C=C bond suggesting that the endo C=C bond is in fact stronger than the exo C=C bond.
The table shows that the absorption frequency of the C=C bond and C-Cl bond is slightly increased in the dihydro derivative of Compound A. The absorption frequencies of the endo C=C and C-Cl did not vary greatly but the exo C=C absorption frequencies did decrease significantly, particularly for the larger substituents. This can be rationalised by the donation of electron density from the occupied p-orbital in the substituent (or π bond in the case of C-CN) into the π* antibonding orbital of the exo C=C bond. This therefore weakens the bond and causes it to absorb at lower frequencies.
Structure based Mini project using DFT-based Molecular orbital methods
Many organic reactions give a mixture of products which are often isomers. Understanding the mechanism by which the reaction proceeds can normally give us a good indication as to which isomers have been formed but conclusive proof of the formation of these isomers must be found. This is usually carried out through analysing NMR spectra and computational chemistry allows the prediction of the 13C NMR spectra of unrecognised molecular systems to a reasonable accuracy.
Synthesis of a Cis-Fused Pyrrolidine
Introduction
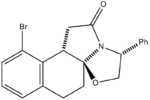
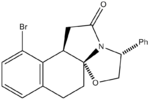
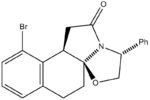
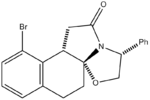
Racemic bicylic lactams can be used in the preparation of non-racemic substituted pyrrolidines. Nitrogen heterocycles occur widely in many natural products and important medicinal synthetic products. This experiment focused on an intermediate in the asymmetric synthesis of U-93385, a potent seratonin-1A agonist. The synthesis involves the simultaneous establishment of two adjacent stereogenic centres through the deracemisation of an achiral ketoester.[4]
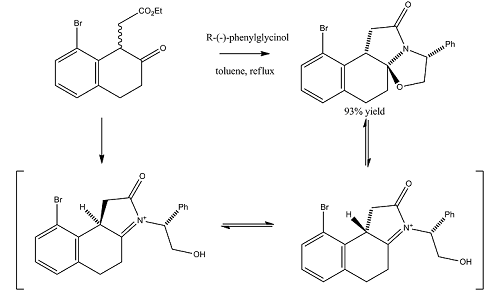
The reaction scheme is shown on the left. As can be seen, the reaction gives a 93% yield of the product stereoisomer even though there are a range of other possible isomers.
Results and Discussion
The geometries of the four isomers were optimised using MM2 in order to find their energies relative to each other. Optimisation using MOPAC/PM6 was also carried out. Ennis et al. speculate that if interconversion between the two intermediates was a facile process, the final intramolecular cyclisation step would occur under thermodynamic control. If this were the case, comparing the relative energies from the MM2 and/or MOPAC/PM6 calculations should allow us to predict the preferred isomer. From the table it can be seen that Isomer B and Isomer C are higher in energy than Compound 1 and Isomer A and this was confirmed by Ennis et al. However Isomer A appears to be of a similar energy to the product and so this isomer cannot be eliminated through thermodynamic considerations.
Analysis of the NMR Chemical Shift calculation
Having optimised the geometry of the molecules using the MOPAC/PM6 method, they were further optimised on the SCAN by running B3LYP/6-31G(d,p). After this had been completed the optimised geometries were used to calculate the predicted 13C NMR spectra for the four isomers. Before the NMR chemical shifts were compared, a few corrections had to be made to the values predicted. The C-Br shift is reduced by -12 ppm and the carbonyl of the amide is corrected by:
δcorr = 0.96δcalc + 12.2.
| Carbon number | Degeneracy | δ/ ppm | Literature δ/ ppm |
|---|---|---|---|
| 7 | 1 | 167.1 | 176.9 |
| 8 | 1 | 165.6 | 139.8 |
| 14 | 1 | 156.0 | 138.7 |
| 10 | 1 | 152.8 | 136.3 |
| 18 | 1 | 138.1 | 131.2 |
| 17 | 1 | 124.2 | 128.8 |
| 9 | 1 | 95.1 | 128.1 |
| 20 | 1 | 75.3 | 127.6 |
| 24 | 1 | 75.0 | 125.5 |
| 22 | 1 | 73.2 | 124.7 |
| 3 | 1 | 72.7 | |
| 4 | 1 | 72.4 | 101.2 |
| 21 | 1 | 71.9 | |
| 23 | 1 | 71.4 | 73.4 |
| 2 | 1 | 69.6 | 57.5 |
| 1 | 1 | 61.3 | 44.9 |
| 6,5 | 2 | 59.4 | 41.1 |
| 19 | 1 | 56.9 | 30.7 |
| 13 | 1 | 32.8 | 27.3 |
Looking at the table of NMR values, it can be seen that unfortunately the predicted NMR chemical shifts do not match up well against the literature values found. The literature values only gave 17 peaks for 20 carbon atoms. This is because there can be problems with the integration in a 13C NMR spectrum and if the two peaks are very close together, they may be amalgamated and counted as one. Small differences in the predicted and expected chemical shifts may be attributed to the proximity of the carbon atom to functional groups other than the cabonyl of the amide, for which corrections had not been made.
| Isomer A, δ/ ppm | Isomer B, δ/ ppm | Isomer C, δ/ ppm |
|---|---|---|
| 167.7 | 166.9 | 165.1 |
| 166.2 | 165.6 | 164.1 |
| 153.2 | 159.0 | 157.5 |
| 147.5 | 153.1 | 152.1 |
| 137.7 | 134.6 | 133.6 |
| 119.6 | 124.9 | 129.2 |
| 97.8 | 97.6 | 96.2 |
| 74.6 | 74.9 | 75.4 |
| 72.9 | 73.3 | 73.3 |
| 72.4 | 72.7 | 72.8 |
| 72.0 | 72.1 | 72.3 |
| 70.8 | 71.5 | 71.4 |
| 69.3 | 71.4 | 69.6 |
| 69.1 | 68.7 | 68.6 |
| 63.2 | 63.4 | 64.3 |
| 60.8 | 62.5 | 63.2 |
| 60.6 | 59.2 | 62.3 |
| 58.7 | 56.8 | 59.1 |
| 32.6 | 15.7 | 17.2 |
Analysis of the 13C NMR spectra does not give conclusive proof of the formation of the particular isomer at all. The large difference in many of the predicted and experimental chemical shifts may be because the molecule adopted a different conformation. The particular molecule was selected for analysis due to the large number of connected rings which would reduce the number of possible conformations the molecule could adopt.
References
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- ↑ P. Camps, F. Perez, S. Vazquez, Tetrahedron, 1997, 53, 9727-9734 DOI:10.1016/S0040-4020(97)00595-4
- ↑ M. Ennis et al. J. Org. Chem, 1996, 61, 5813-5817 DOI:10.1021/jo960185l