Rep:Mod:jwy17
NH3
Link to .log file https://wiki.ch.ic.ac.uk/wiki/images/c/c2/JY_OPT_NH3_1.LOG
Bond Length
Each N-H = 1.01798 Angstrom
Bond Angle
H-N-H angle = 105.741 degrees
nh3 optimisation File Name JY_opt_nh3_1 File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -56.55776873 a.u. RMS Gradient Norm 0.00000485 a.u. Imaginary Freq 0 Dipole Moment 1.8466 Debye Point Group C3V
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986286D-10
Optimization completed.
-- Stationary point found.
NH3 |
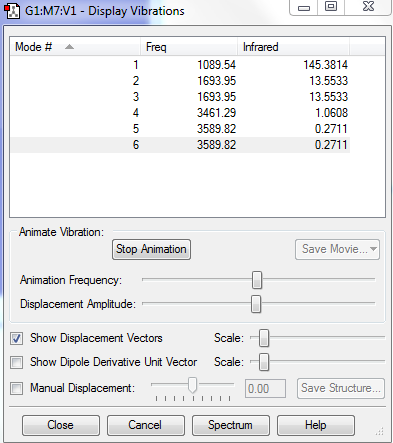
Vibrations
-Using the 3N-6 rule, the expected number of vibrational modes is 6, which is true based on the Gaussian program.
-There are 2 pairs of vibrational modes that are degenerate.
-3 vibrational modes (the first three in the above picture) are bending vibrations and 3 other are stretching vibrations.
-2 of these vibrational modes are highly symmetric
-1089.54 HZ is the 'umbrella' mode.
-4 bands should appear because there are 2 pairs of degenerate vibrational modes.
Charge Distribution Over NH3 Molecule
we would expect the N atom to have the partial negative charge of -1.125 and H atams to have partial positive charge of +0.375. As the N atom is much more electronegative than the 3 H atoms, it 'pulls' electron density towards itself and equally from all 3 H atoms, which is why the magnitude of charge on N is 3 times larger than the Hs'.
HOMO and LUMO of NH3
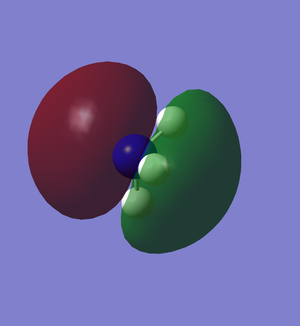
The HOMO of NH3 is the deconstructive overlapping of the nitrogen's s orbital and the Hs' s orbitals all in the same phase. Hence, it is an antibonding molecular orbital.
H2 and N2
H2
Link to .log file https://wiki.ch.ic.ac.uk/wiki/images/2/2e/JY_OPT_H2_1.LOG
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES Predicted change in Energy=-5.726834D-09 Optimization completed. -- Stationary point found.
File Name JY_opt_h2_1 File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -1.17853935 a.u. RMS Gradient Norm 0.00003809 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D*H
H2 |
N2
Link to .log file https://wiki.ch.ic.ac.uk/wiki/images/4/4b/JY_OPT_N2_1.LOG
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401111D-13 Optimization completed. -- Stationary point found.
File Name JY_opt_n2_1 File Type .log Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -109.52412868 a.u. RMS Gradient Norm 0.00000060 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D*H
N2 |
Calculation for energy for Haber-Bosch reaction
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.11553746 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853935 a.u.
3*E(H2)= -3.53561805 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579073 a.u.
-0.05579073/2 a.u. = -73.24 kJ/ mol (2d.p.)
CF4
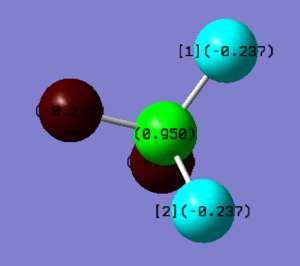
Charge Distribution
The C atom (centre) has a charge of +0.950 and the F atoms (highlighted blue or red) has a -0.237 charge
Vibrational modes
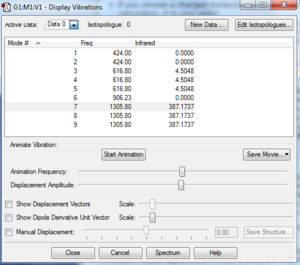
There are 2 types vibrations with 3 degenerate levels as the CF4 molecule vibrates similarly but in 3 different directions. Using the 3N-6 rule, the expected number of vibrational modes is 9, which the computer predicted as well. The are 3 vibrational modes that do not contribute to the infrared spectrum. This suggests that these 3 vibrational mode are symmetrical and do not produce dipole moments.
Molecular orbitals of CF4
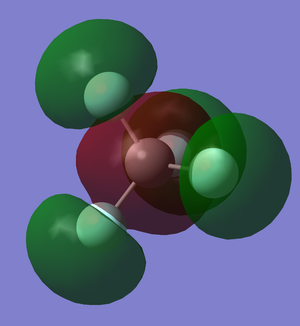
C-F sigma bond
This molecular orbital is formed by the overlap of the 2p orbitals of the fluorine atoms and the 2s orbital of the carbon atom . As this molecular orbital is filled, this molecular orbital contribute 1 bond order to the overall molecule.
2 s orbitals overlap
A very simple molecular orbital formed by the overlapping of the 2s orbitals of each atom. This is not the 1s orbital overlap as the 1s electrons in fluorine are too deep in energy to interact with other atomic orbitals.
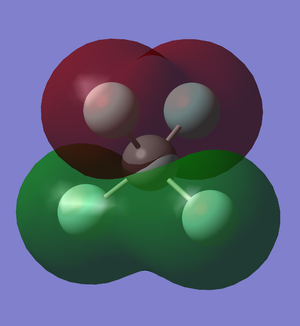
Fluorine 2s overlap
This molecular orbital does not involve the atomic orbitals of the carbon atoms. Instead, it is the overlapping of the 2s orbitals of 2 fluorine atoms and is similar for the other 2 fluorine atoms but with different phases.
Fluorine 2P overlap 'hugging'
This molecular orbital, like the previous one, does not involve orbitals from the carbon itself. In this molecular orbital, all the 2p orbitals are aligned and such that the one whole side of the molecule is the opposite phase of the other side. The result shows the 2 opposite-phased molecular orbital seemingly hugging each other.
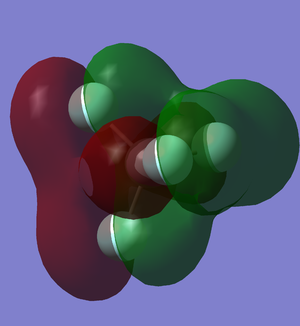
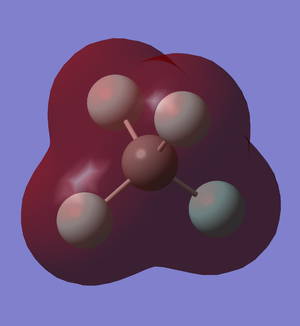
LUMO of CF4
After the carbon atoms form 4 bond with the fluorine atoms, the carbon's 2s and 2p orbital are used and occupied.