Rep:Mod:juneywiki
Conformational Analysis using Molecular Mechanics (Part 1)
The Hydrogenation of a Cyclopentadiene Dimer
The dimerisation of cyclopentadiene is a [4+2] cycloaddition reaction. We can predict the stereoselectivity of the dimer product using the Woodward-Hoffmann rules, and therefore the stereochemistry of the dimerisation transition state; the reaction proceeds thermally via a Huckel (suprafacial) transition state or photochemically via a Mobius (antarafacial) transition state.
However, there are two possible products of this reaction depending on the orientation of the cyclopentadiene molecule (see Figure 1). The exo- (1) and endo- (2) products arise from the orientation of a second cyclopentadiene molecule facing "away" or "towards" the first. The Alder rule dictates that the endo-product is the major product, due to the favourable orbital overlap between the molecules - an overlap that is absent in the exo- transition state[1].
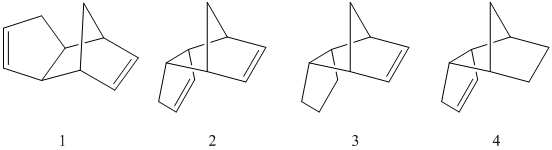
Conformational analysis using molecular mechanics has been conducted on:
- the cyclopentadiene exo dimer (1)
- the cyclopentadiene endo dimer (2)
- the hydrogenation products of the endo dimer - norborene (3) and cyclopentene (4)
using the MM2/MMFF94 force field. The results are presented below.
| Energy Contributions | 
|
|
|
|
| Exo-product (1) | Endo-product (2) | Norborene (3) | Cyclopentene (4) | |
| Bond Stretching | 3.54268 | 3.46746 | 3.30836 | 2.82294 |
| Angle Bending | 30.77252 | 33.19101 | 30.86423 | 24.68546 |
| Stretch Bending | -2.04121 | -2.08217 | -1.92652 | -1.65712 |
| Torsional | -2.72821 | -2.94964 | 0.05942 | -0.3782 |
| Out-of-plane Bending | 0.01489 | 0.02196 | 0.01536 | 0.00028 |
| Van der Waals | 12.79923 | 12.35754 | 13.281 | 10.6371 |
| Electrostatic | 13.01361 | 14.18453 | 5.12099 | 5.14702 |
| Total | 55.37351 | 58.19069 | 50.72284 | 41.25748 |
From this computational analysis we find that the endo dimer is higher in energy than the exo dimer by 2.8 kcal/mol. However, we also know that the endo-product is favoured. This therefore suggests that reactivity is governed by kinetic rather than thermodynamic factors.
We also calculate that the favoured hydrogenation product is (4) rather than (3), which is in accordance with the literature[2]. The results suggest that the reaction is under thermodynamic control, as (4) is lover in energy than (3) by roughly 9.4 kcal/mol. We see the main contributions towards the difference in energy are due to angle bending and van der Waals, suggesting that the cyclopentene product is the less sterically hindered of the two.
The Taxol Intermediates
Taxol is an important drug typically used in the treatment of ovarian, breast and lung cancers amongst others[3]. The intermediate in this study is a key intermediate identified by Paquette[4], and is a good demonstration of atropisomerism.
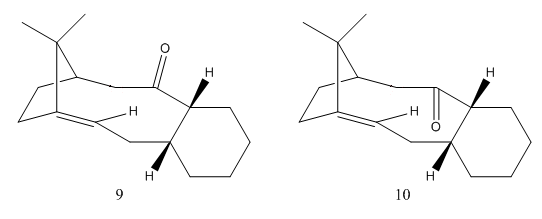
Atropisomerism arises from hindered rotations, which results in two or more conformations that correspond to different isomers. In this molecule, the barrier to rotation is from the C-C bond between the 6- and 10-membered rings, which means that the carbonyl must occupy either the same or opposite face as the isopropyl bridge. Since the conformation of the smaller ring determines that of the larger ring[5], this results in eight conformers - 4 conformational minima are known for a 6-membered ring, and there are two orientations of ketone.
The ketones were drawn and minimised in Avogadro using the MMFF9s force field, and the results given below.
| Energy Contributions | 
|
|
| Carbonyl Down | Carbonyl Up | |
| Bond Stretching | 7.14282 | 7.52866 |
| Angle Bending | 22.42601 | 21.53112 |
| Stretch Bending | -0.24228 | 0.012 |
| Torsional | -1.91312 | -0.51744 |
| Out-of-plane Bending | 0.66967 | 0.93772 |
| Van der Waals | 32.12172 | 33.49379 |
| Electrostatic | 0.82973 | 0.27425 |
| Total | 61.03455 | 63.2601 |
Only one chair per ketone was computed, however the other energies may be found by changing the conformation of the ring. Chairs are expected to be lower energy than twist-boats, which in turn are lower than the proposed transition state boat and half-chair structures.
Comparison of the two atropisomers allows us to determine which is formed by the oxy-Cope reaction under equilibrating conditions, the one that is most thermodynamically stable will be preferentially formed. (9) is more stable than (10), but only by 2.2 kcal/mol, which would suggest that a mixture forms under equilibrating conditions with (9) being the major component in the mixture.
Hyperstability
Bredt's rules states that double bonds at ring junctions are unstable, which leads to enhanced reactivity. However, molecule (10) is only fully hydrogenated after extended periods of time[6]. Therefore we need to investigate the relative energies of the alkene and alkane.
| Bond Stretching | 7.14282 | 7.21955 |
| Angle Bending | 22.42601 | 24.73421 |
| Stretch Bending | -0.24228 | 0.01292 |
| Torsional | -1.91312 | 6.23478 |
| Out-of-plane Bending | 0.66967 | 0.81524 |
| Van der Waals | 32.12172 | 31.89353 |
| Electrostatic | 0.82973 | 0.00163 |
| Total | 61.03455 | 70.91186 |
The energy of the alkane was computed using the MMMFF9s force field and Conjugate Gradients method, and it is seen to be less stable than alkene (10) by nearly 10 kcal/mol. However we expect that the alkane would be more stable than the bridghead alkene. This discrepancy can be mainly attributed to the difference in the torsional contribution, whichsuggesets that the conformation adopts a partially eclipsed structure.
Another factor to consider is olefinic strain, which can be calculated by subtracting the total strain of the alkane from the total strain of the alkene. In this case, the olefinic strain is negative, indicating that the enthalpy of hydrogenation is favourable and supporting the relative instability of the alkane to the alkene.
Spectroscopic Simulation using Quantum Mechanics
Avogadro was first used to optimise the energies of ketones (17) and (18) using the MMFF9s force field.

| Energy Contributions |  |
 |
 |
 |
| Carbonyl Up (Chair) | Carbonyl Up (Twist Boat) | Carbonyl Down (Chair) | ||
| Bond Stretching | 16.57396 | 15.22508 | 15.07066 | 14.39859 |
| Angle Bending | 30.13766 | 29.15943 | 30.8648 | 28.1791 |
| Stretch Bending | 0.237 | 0.39803 | 0.60292 | 0.44557 |
| Torsional | 12.18456 | 14.35533 | 9.7361 | 13.98699 |
| Out-of-plane Bending | 0.99425 | 1.07303 | 0.90623 | 1.04101 |
| Van der Waals | 52.77833 | 52.65604 | 49.39199 | 50.54797 |
| Electrostatic | -7.66268 | -7.40526 | -6.10377 | -6.37105 |
| Total | 105.24308 | 105.46168 | 100.46893 | 102.22818 |
The predicted NMR spectra for ketones 17 and 18 are shown below. The reference is TMS and the solvent is benzene.
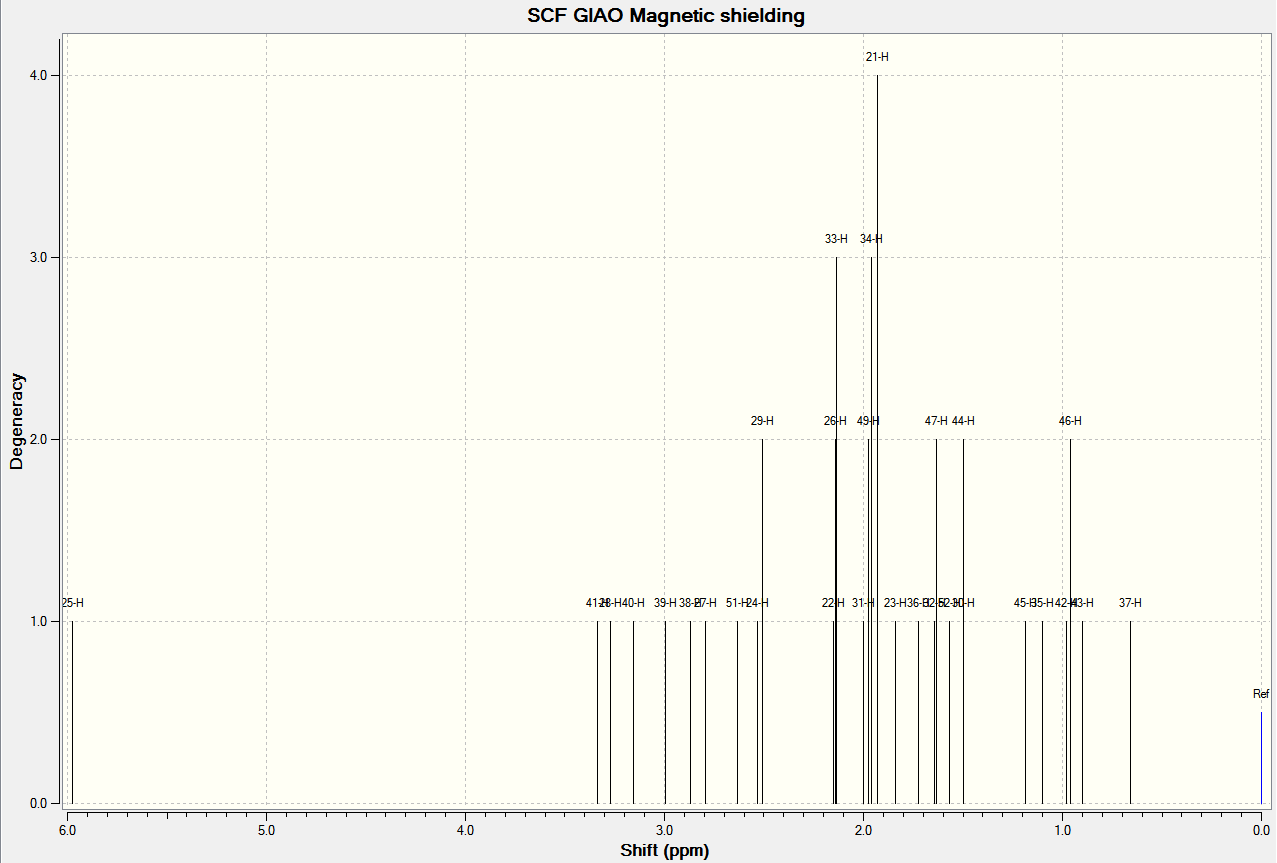
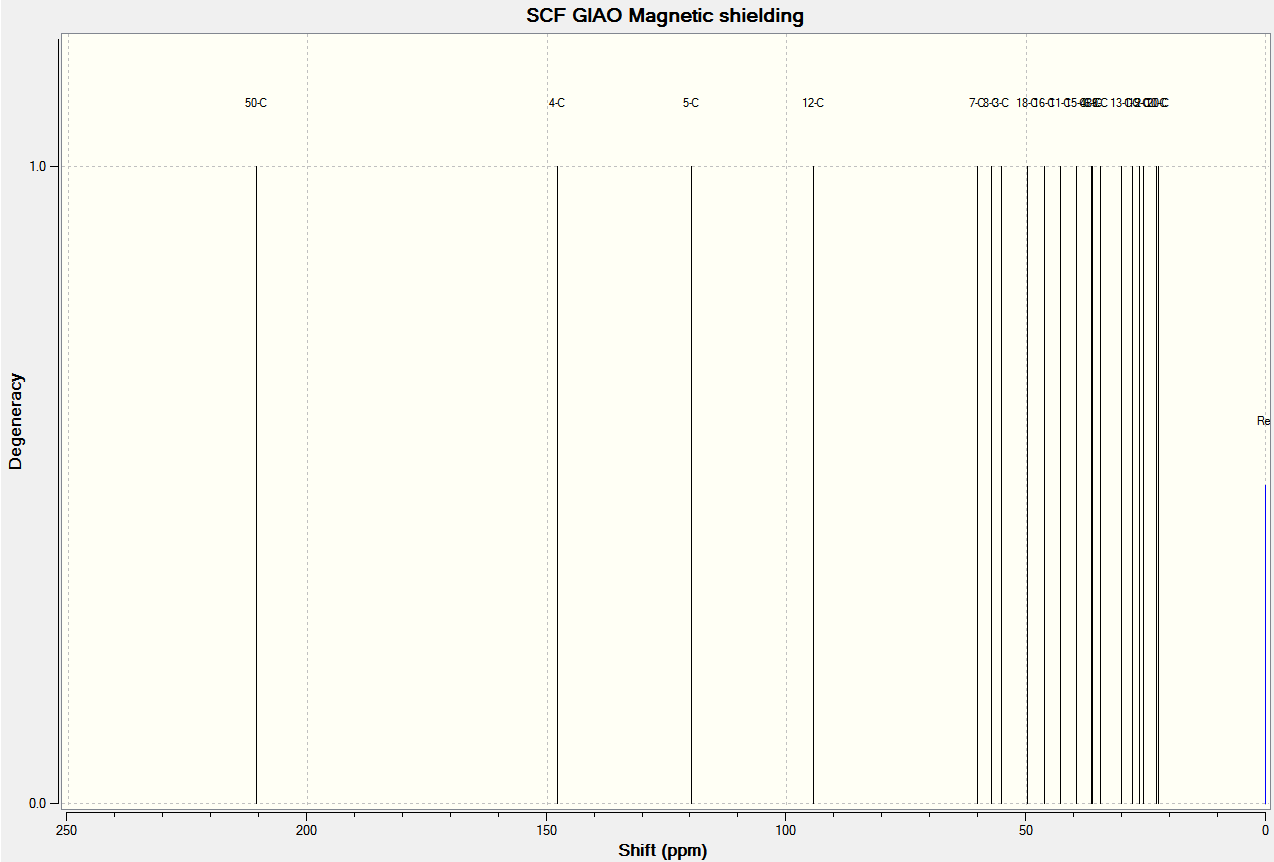


Analysis of the properties of the Synthesised Alkene Epoxides (Part 2)
The Shi Asymmetric Catalyst
Crystal Structure
The Jacobsen Asymmetric Catalyst
Crystal Structure
The Products - Alkene Epoxides
Calculated NMR Properties
Absolute Configurations
Optical Rotation paragraph
Chiroptical Properties paragraph
Using the (calculated) properties of transition state for the reaction (β-methyl styrene only
Shi catalyst
Jacobsen catalyst
Other Alkenes
Investigating the non-covalent interactions in the active-site of the reaction transition state
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
Suggesting new candidates for investigations
References
- ↑ R. Hoffmann, R.B. Woodward, J. Am. Chem. Soc., 1965, 87, 4388
- ↑ D. Skála, Petroleum and Coal, 2003, 45, 105-108
- ↑ M.W. Saville; J. Lietzau; J.M. Pluda; W.H. Wilson; R.W. Humphrey; E. Feigel; S.M. Steinberg; S. Broder, et al. The Lancet , 1995, 346, 8966
- ↑ L. Paquette, S. Elmore, Tetrahedron Letters, 1991, 32, 319-322
- ↑ W. Maier, P. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891-1900
- ↑ W. Maier, P. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891-1900
