Rep:Mod:jun0404
- Moleclar Orbital Calculations
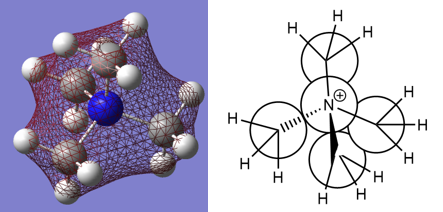
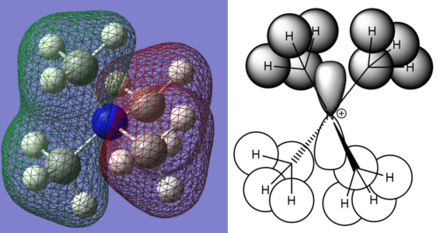
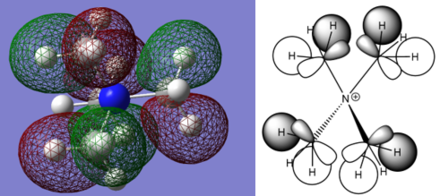
Charge Distribution Calculations
A full NBO charge analysis was performed with a range of -0.6 to +0.6
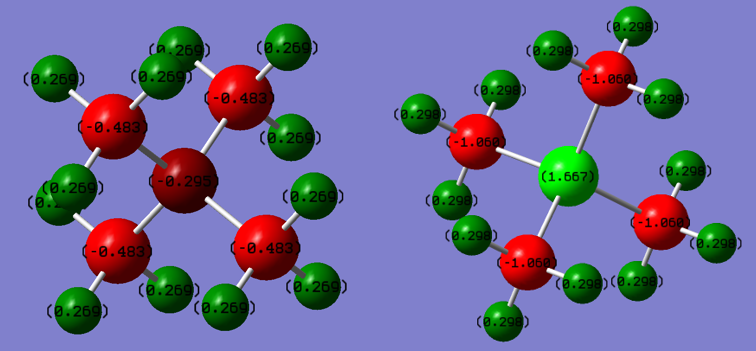
RIGHT: Charge distribution for [P(CH3)4]+.
[N(CH3)4]+ charges(DEBYE):
- Nitrogen: -0.295
- Hydrogen: 0.269
- Carbon: -0.483
[P(CH3)4]+ charges(DEBYE):
- Phosphorous: 1.667
- Hydrogen: 0.298
- Carbon: -1.060
The formal charge on the Nitrogen or Phosphorous is merely a convention, assuming
that electrons in all chemical bonds are shared equally between atoms, regardless of
relative electronegativity. It is also assumed that electrons are localised in the bonds
and thus formal charges in the valence bond model keep track of the electrons around the
atom, in this case N or P. However, as shown in both molecules the charge is delocalised
in the entire molecule .
Specifically in [N(CH3)4]+ the positive charge is found on the electropostive protons, while
the nitrogen, being an electronegative element is negatively charged. This is the opposite of what
of what VSEPR and localisation of bonds predict, exactly because in real molecules, molecular
orbitals that span all over the entire molecule are present, leading to delocalisation of the
charge.
When compared to [N(CH3)4]+ , the charge separation in the C-P bond is greater than in the N-P bond
due to the greater difference in electronegativity between C&P(𐤃elec=0.49) and C&N(𐤃elec=-0.36). Also
the charge on the phosphorous is now positive since phosphorous is more electropostive than nitrogen.
Therefore in this case the charge is greatly found on the phosphorous atom and partly in the hydrogens.
