Rep:Mod:js71ckl10
Week 1
Cyclopentadiene Dimer
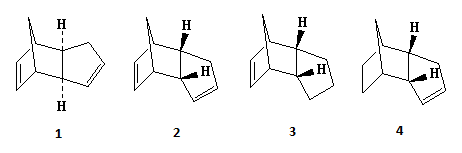
The dimerisation and hydrogenation[1] of cyclopentadiene were investigated in this experiment. By looking at the characteristics of the dimers, one can predict the thermodynamics of the reaction. The diagram below shows the dimers under investigation. The results obtained through minimising the energy of the dimers using ChemBio3D Ultra (MM2 force field) are shown in the table below.
|
Parameter |
Energy Difference |
Energy Difference |
||||
|
Stretch |
1.2859 |
1.2508 |
0.0351 |
1.2772 |
1.1301 |
0.1471 |
|
Bend |
20.5800 |
20.8501 |
0.2701 |
19.8592 |
13.0150 |
6.8442 |
|
Stretch-Bend |
-0.8382 |
-0.8355 |
0.0027 |
-0.8345 |
-0.5652 |
0.2693 |
|
Torsion |
7.6557 |
9.5102 |
1.8545 |
10.8093 |
12.4113 |
1.602 |
|
Non-1,4 VDW |
-1.4206 |
-1.5645 |
0.1439 |
-1.5627 |
-1.0520 |
0.5107 |
|
1,4 VDW |
4.2323 |
4.3179 |
0.0856 |
5.6334 |
4.4405 |
1.1929 |
|
Dipole/Dipole |
0.3775 |
0.4475 |
0.07 |
0.1621 |
0.1410 |
0.0211 |
|
Total energy |
31.8765 |
33.9975 |
2.121 |
35.6850 |
29.2475 |
6.4375 |
The dimerisation of cyclopentadiene can result in two isomers, exo dimer (molecule 1) and endo dimer (molecule 2). The endo dimer is higher in energy by 2.121 kcal/mol compared to the exo dimer. The main contribution to the energy difference is torsion. The endo dimer has a larger torsion energy, difference of 1.8545 kcal/mol, compared to the exo dimer. Larger torsion energy of the exo dimer suggests that the groups have larger angles between them thus the isomer is more staggered (antiperiplanar) compared to the endo isomer. The more staggered conformer is favoured over the more eclipsed conformer as there is less steric repulsions between groups and it could lead to stabilisation by allowing sigma-sigma* overlap. Therefore the exo dimer is more thermodynamically stable and has a lower energy. Despite this, the endo dimer is the isomer that is formed during dimerisation of cyclopentadiene. This means that the reaction must be kinetically controlled, since the more thermodynamic stable product (exo-dimer) is not formed. Some might argue that the reason for this is due to secondary orbital interactions, with the endo isomer having additional stabilisation due to the interactions between occupied and unoccupied orbitals. This lowers the activation barrier of the endo isomer which has extra stabilisation energy that the exo isomer does not posse. However this does not fully account for the observed trend and the full explanation is far beyond the scope of the course [2].
The hydrogenation of cyclopentadiene could result in molecules 3 or 4.
Molecule 4 has a lower energy, difference of 6.4375 kcal/mol, compared
to molecule 3. The main contribution to their energy difference
is the bending energy, with molecule 3 being higher by 6.8442 kcal/mol compared to molecule 4. In cyclopentadiene dimer, the double bonds in the 5-membered rings have unfavourable bond angles (ideally, 120 for sp2 atoms) which is the cause of this bending energy. Hydrogenation across the double bond can relieve the bending energy. The major (or only) product formed would be the hydrogenation of the more-strained double bond as this would lead to greater decrease in strain and energy of the molecule. One of the 5-membered rings consists of a bridge leading to another aromatic ring. The double bond in this ring has a higher strain compared to the other one so this is the double bond that is hydrogenated. This leads to molecule 4 with the lower bending and total energy and molecule 3 is only formed after prolonged hydrogenation.
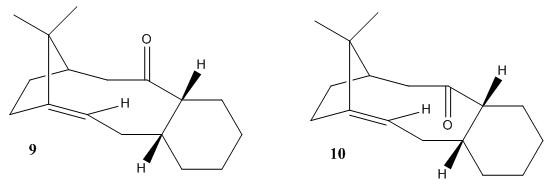
Atropisomers are formed when the rotation about single bonds is hindered and the steric strain barrier to rotation is high enough to allow for the isolation of the conformers. The Oxy-Cope rearrangement proposed by Paquette[3] indicated intermediates which are atropisomers for Taxol synthesis. The intermediates are molecules 9 and 10 which differ by the direction of the carbonyl bond. The more stable intermediate can be found by performing calculations of energy minimisation with MM2 forcefield and results are shown in table below.
|
Parameter |
Energy Difference |
||
|
Stretch |
3.2301 |
2.6209 |
0.6092 |
|
Bend |
17.2721 |
11.3431 |
5.929 |
|
Stretch-Bend |
0.2704 |
0.3433 |
0.0729 |
|
Torsion |
18.2111 |
19.6657 |
1.4546 |
|
Non-1,4 VDW |
0.3309 |
-2.1601 |
1.8292 |
|
1,4 VDW |
13.8090 |
12.8724 |
0.9366 |
|
Dipole/Dipole |
-1.6917 |
-2.0023 |
0.3106 |
|
Total energy (kcal mol-1) |
51.4318 |
42.6829 |
8.7489 |
MMFF94 force field can also be used which takes into account different interactions. The resulting energy is different to that of the MM2. However the same trend is observed, with molecule 10 being more stable than molecule 9.
|
Total Energy |
Molecule 9 |
Molecule 10 |
Energy Difference |
|
MM2 |
51.4318 |
42.6829 |
8.7489 |
|
MMFF94 |
76.2713 |
60.5581 |
15.7132 |
After performing both calculations, it can be seen that the “down” isomer is more stable, by 8.7489 kcal/mol (MM2) and 15.7132 kcal/mol (MMFF94), compared to the “up” isomer. The main contribution to the difference in energy is the bending energy, with “up” isomer being larger by 5.9290 kcal/mol compared to the “down” isomer. Therefore the “down” isomer is the thermodynamically more stable product and should be the major product under thermodynamic conditions. The six membered ring in both isomers can adopt different conformations and these are investigated. Twist boat conformations are less stable than the respective chair conformations and this can be seen by the increased in energy in the twist boat conformations (for both MM2 and MMFF94 force field) by comparing tables immediate above and below. It is worth mentioning that the 9a twist boat conformer is still higher in energy than the 10a twist boat conformer, further validating that carbonyl “up” is an unfavourable conformation. The relatively large energy difference between the chair and twist boat conformers suggests that it is difficult to twist the aromatic ring. The steric strain barrier to rotation is high enough to allow for the isolation of the conformers, forming atropisomers.
|
Total Energy |
Energy Difference |
||
|
MM2 |
57.4953 |
47.0648 |
10.4305 |
|
MMFF94 |
82.9969 |
66.7961 |
16.2008 |
Regioselective Addition of Dichlorocarbene to a diene
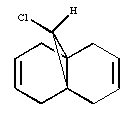
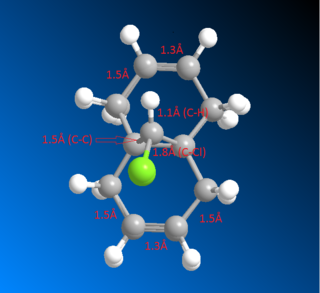
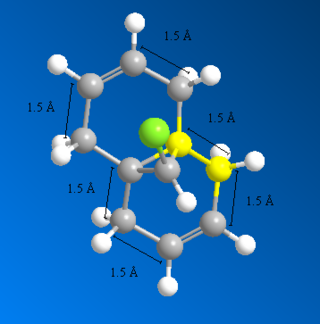
Overlay showing the bond distances of the molecule 12 above computed using MM2 DOI:10042/22251 and MOPAC DOI:10042/22250 :
After inspecting the the actual bond distances of the molecules minimised by MM2 and MOPAC, it can be shown that the bonds indicated below with 5Å have the largest differences between the two molecules (around 0.01Å). This suggests that the C-C bonds that are not shown in the diagram, ie C-C single bridging, C=C double and C-C single bond that connects the two aromatic ring together, are very fixed and non-flexible. Because MM2 and MOPAC takes into account different interactions in the molecule and both calculations yielded pretty much the exact same distance.
The vibrations of molecule 12 is also studied. The most important ones are outlined below.
|
Frequency |
Intensity |
Form of Vibration |
Animation |
|
770.86 |
25.1312 |
C-Cl stretch |
|
|
1753.04 |
3.99363 |
C=C exo |
|
|
1757.34 |
3.9363 |
C=C endo |
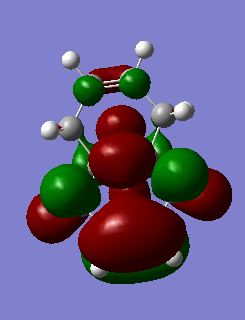
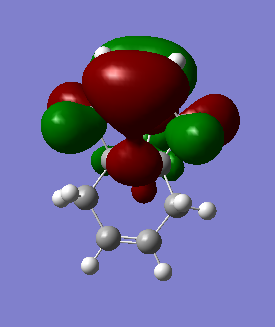
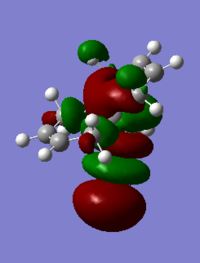
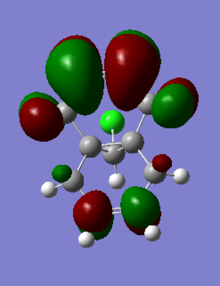
The table below shows the molecular orbitals of molecule 12[4]. In HOMO-1, electron density can be seen in both double bonds, with the double bond that is exo to C-Cl having much larger lobes, i.e. it is more electron rich and more prone to nucleophilic attack. In HOMO however, the opposite is observed where larger lobes are seen on the double bond that is endo to C-Cl has and the other double bond has no electron density around it at all. This is the reason why carbenes attack the endo double bond instead of the exo double bond. In LUMO, the number of nodal planes has clearly increased and energy has changed from negative to positive, showing the increase in anti-bonding character. Orbitals are also smaller so they are less diffused. LUMO+1 has even more anti-bonding character in the MOs with higher energy and no electron density can be seen in the exo double bond. The trend continues to LUMO +2, where no large or diffused electron density can be seen and there is no electron density around the middle bridge and the Cl atom.
|
MO |
Energy |
MO picture |
|
HOMO-1 |
-0.24519 |
|
|
HOMO |
-0.23588 |
|
|
LUMO |
0.01555 |
|
|
LUMO+1 |
0.02025 |
|
|
LUMO+2 |
0.03613 |
Monosaccharide chemistry and the mechanism of glycosidation
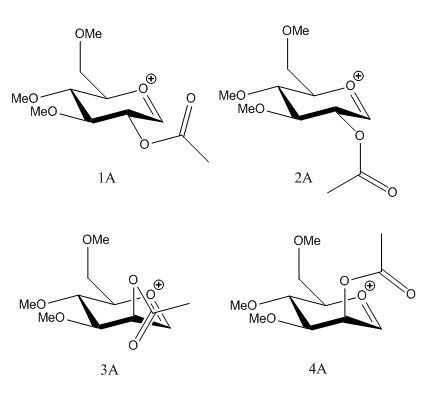
The first step of glycosidation involves the loss of a leaving group at the anomeric carbon. The resulting oxonium intermediate is planar so there is equal probability of SN1 nucleophilic attack from top and bottom face. The neighbouring group effect can control the regioselectivity issue by essentially blocking the attack of nucleophile from one face, which leads to the formation of the trans-product5. Firstly, four conformers of the lowest energy found were investigated. Energy was minimised using MM2 and heat of formation was also found using MOPAC.
|
Parameter |
Energy Difference |
Energy Difference |
||||
|
Stretch |
2.1897 |
2.2463 |
0.0566 |
2.4135 |
2.5136 |
0.1001 |
|
Bend |
10.5951 |
9.8645 |
0.7306 |
11.5412 |
14.8334 |
3.29228 |
|
Stretch-Bend |
0.8251 |
0.8005 |
0.0246 |
0.9671 |
1.0882 |
0.1211 |
|
Torsion |
1.3840 |
1.3783 |
0.0057 |
0.3157 |
1.9530 |
1.6373 |
|
Non-1,4 VDW |
-3.8012 |
-2.9338 |
0.8674 |
-1.5602 |
-1.5066 |
0.0536 |
|
1,4 VDW |
18.9398 |
19.2928 |
0.353 |
18.0160 |
17.7991 |
0.2169 |
|
Charge/Dipole |
-0.4433 |
-11.0357 |
10.5924 |
-10.0768 |
4.8648 |
14.9416 |
|
Dipole/Dipole |
4.4460 |
5.9075 |
1.4615 |
7.1479 |
5.8223 |
1.3256 |
|
Total energy |
34.1352 |
25.5203 |
8.6149 |
28.7644 |
47.3679 |
18.6035 |
|
Energy |
Molecule 1A |
Molecule 2A |
Energy Difference |
Molecule 3A |
Molecule 4A |
Energy Difference |
|
Heat of Formation |
-66.72260 |
-67.48977 |
0.76717 |
-66.66579 |
-56.78597 |
9.87982 |
1A and 2A have the acetyl group at the equatorial position and 3A and 4A have the acetyl group at the axial position. The acetyl group in 1A and 4A point away from the aromatic ring and it points towards the aromatic ring in 2A and 3A. For the two equatorial acetyl conformers, the one that points towards the ring is lower in energy; 2A is lower in energy by 8.6149 kcal/mol compared to 1A. This is also true for the axial acetyl conformers. Acetyl group pointing towards the ring shows extra stabilisation; 3A is lower in energy by 18.6035 kcal/mol compared to 4A. This can be due to increased orbital interactions between the acetyl group with the oxonium cation. Another possible explanation is related to charge dipoles. The closer the acetyl group to the oxonium cation, the smaller the charge dipole thus molecule is more stabilised. For the two equatorial isomers, 1A has a larger charge dipole compared to 2A so 1A is less stable, hence it has a greater energy. The same can be observed from the axial isomers, with 3A having a more stable charge dipole energy compared to 4A, which has a higher energy. The MOPAC calculations also agrees with that of the MM2, with 2A and 3A being lower in energy compared to 1A and 4A respectively.
The table below shows the distances between the O atom in the acetyl group and the anomeric C atom. Clearly for the less stable structures (1A and 4A), the distances are larger than that of the more stable isomers (2A and 3A). Larger distances means increased difficulty of attack from the oxygen atom to the anomeric C atom. The increased distances may also contribute to lower orbital interactions and higher charge dipoles, both of which are the causes of the increased in energy.
|
Parameter |
1A |
1B |
1C |
1D |
|
Bond Distance |
3.9 |
1.7 |
1.6 |
4.3 |
1A
1A |
1B
1B |
1C
1C |
1D
1D |
The intermediate where the carbonyl oxygen has formed a bond to the anomeric carbon is also investigated. The four conformers, 1B, 2B, 3B and 4B (lowest energy isomers that i could find), also show similar trend to the previous isomers. This is 2B and 3B being more stabilised compared to 1B and 4B respectively, although the energy difference is less than that observed from above. Again, the charge dipoles of 2B and 3B are smaller than 1B and 4B respectively, leading to lower total energy of the molecule. MOPAC calculation also shows the same trend, with 2B and 3B being lower in energy compared to 1B and 4B. Having investigated the reactant and product energies, it can be concluded that the neighboring group effect is not favourable for 1A and 4A as both of these and their resulting products have higher energy. Therefore the formation of the β-anomer (trans product) is very favourable due to lower energy (ie 2A and 3A).
|
Parameter |
Energy Difference |
Energy Difference |
||||
|
Stretch |
2.6510 |
2.4321 |
0.2189 |
2.5825 |
2.6681 |
0.0856 |
|
Bend |
17.4406 |
16.2919 |
1.1487 |
16.6213 |
17.0389 |
0.4176 |
|
Stretch-Bend |
0.7645 |
0.5937 |
0.1708 |
0.7165 |
0.7341 |
0.0176 |
|
Torsion |
7.6820 |
5.5953 |
2.0867 |
6.9043 |
6.0528 |
0.8515 |
|
Non-1,4 VDW |
-2.6350 |
-2.9863 |
0.3513 |
-2.6800 |
-2.3999 |
0.2801 |
|
1,4 VDW |
19.3863 |
17.7296 |
1.6567 |
19.3842 |
19.0560 |
0.32282 |
|
Charge/Dipole |
4.4279 |
-3.1189 |
7.5468 |
-2.5800 |
1.5020 |
4.0820 |
|
Dipole/Dipole |
-0.5301 |
-4.1735 |
3.6434 |
-0.8757 |
0.2787 |
0.5970 |
|
Total energy |
41.6404 |
39.9107 |
1.7297 |
40.0721 |
44.9307 |
4.8586 |
|
Energy |
Molecule 1B |
Molecule 2B |
Energy Difference |
Molecule 3B |
Molecule 4B |
Energy Difference |
|
Heat of Formation |
-66.72260 |
-67.48977 |
0.76717 |
-66.66579 |
-56.78597 |
9.87982 |
week 2
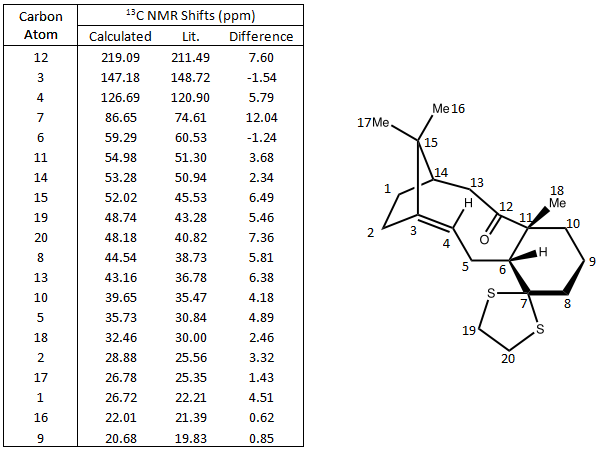
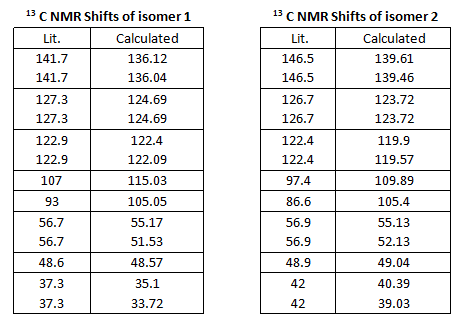
13C NMR Prediction
13C and Taxol derivative (molecule 18) was predicted and compared against literature values. The calculated chemical shifts generally fit well with data. The biggest error is at carbon number 7, which is bonded to the dithiane group. Other chemical shifts are mostly within 5ppm, except for some special cases such as the carbonyl carbon and those bonded to one S atom. DOI:0042/22255
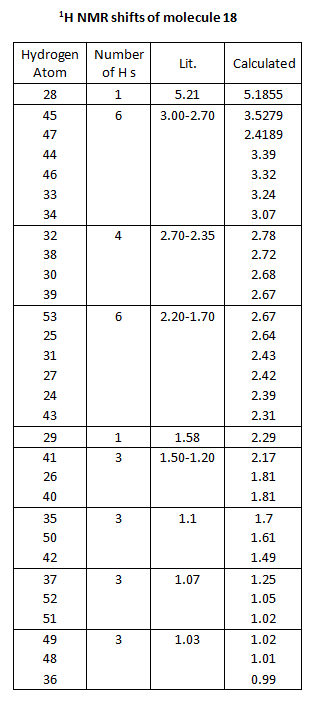
1H NMR Prediction
Picture showing H numbers:
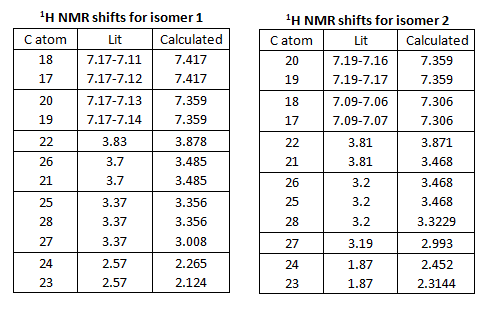
Table showing H numbers, literature and calculated shifts:
The calculated and the literature values of the chemical shifts correspond well. A lot of the predicted values are slightly larger than that of the literature.
Literature Project
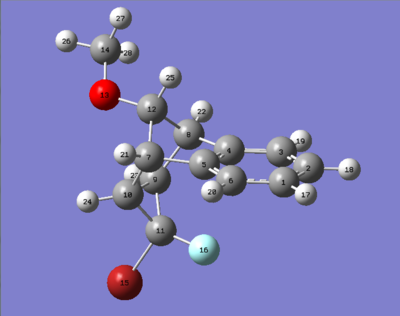
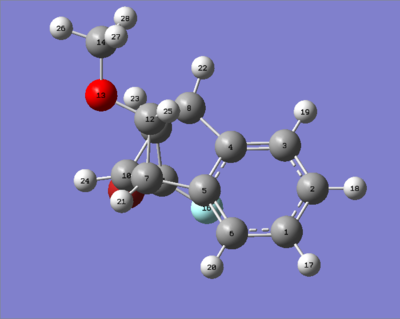
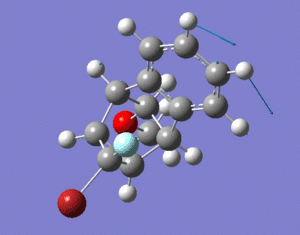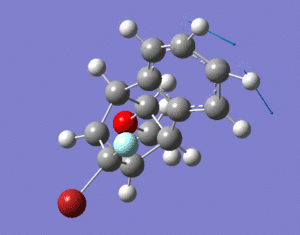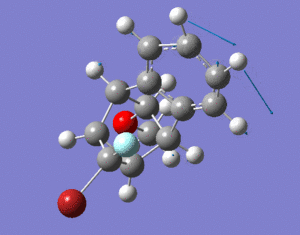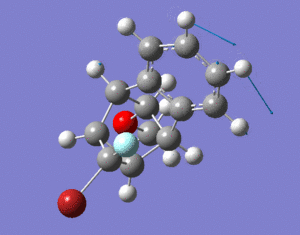
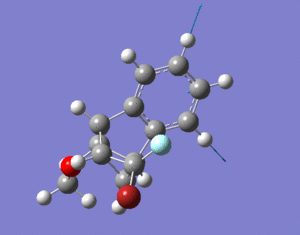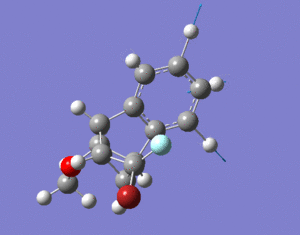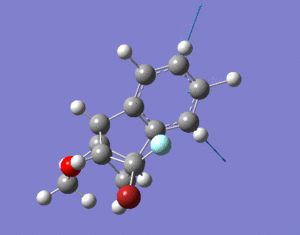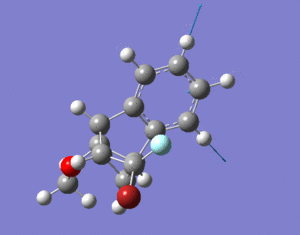
The two isomers shown below were investigated in this project. The aromatic structure consists of two halogens, Br and F. The way these halogens are arranged determine the structure of the isomers. Isomer 1 is when Br is under the aromatic ring and isomer 2 is when F is under the aromatic ring. Energy minimisation calculations were performed using MM2, MMFF94 and MOPAC[2].

isomer 1
1A |
isomer 2
1A |
|
Parameter |
Energy Difference |
||
|
Stretch |
2.6111 |
2.3838 |
0.2273 |
|
Bend |
34.7915 |
30.2498 |
4.5417 |
|
Stretch-Bend |
-1.6059 |
-1.5350 |
0.0709 |
|
Torsion |
2.9995 |
3.1306 |
-0.1311 |
|
Non-1,4 VDW |
-0.9756 |
--1.7219 |
0.7463 |
|
1,4 VDW |
10.8740 |
11.9443 |
-1.0703 |
|
Dipole/Dipole |
0.6264 |
0.6260 |
0.0004 |
|
Total energy (kcal mol-1) |
49.3210 |
45.0774 |
4.2436 |
|
Isomers/Energy |
MMFF94 |
Heat of Formation |
|
Isomer 1 |
97.0042 |
-9.36096 |
|
Isomer 2 |
92.9239 |
-10.25135 |
From these data it can be seen that isomer 1 has higher energy than isomer 2. The biggest contribution to the difference in energy is bending energy; isomer 1 is higher than isomer 2 by 4.5417 kcal/mol. This can be due to the slight increase in steric clash between the larger Br atom and the aromatic structure. The increase in repulsion increases the angle of the C-C-Br bond to 129 deg (shown in diagram below). The C-C-F bond angle is smaller than that (125 deg) as it experiences less repulsion, which is closer to the idea angle 120 deg. The larger the bond angle the more strain the molecule has and so isomer 1 is higher in energy. The MMFF94 and MOPAC calculations all agree with that of MM2, with isomer 1 being higher in energy compared to isomer 2.
Another conformer (press to see mol file) was found with energy 48.1659 kcal/mol. Other conformations only differ in energy by 1 d.p. therefore is not included here. The details of the conformer is outlined below. Its bending energy is in between that of the two isomers above, and so is the total energy. This indicates the importance of bending energy has on the total energy of the molecule.
Stretch: 2.3671 Bend: 33.9513 Stretch-Bend: -1.4393 Torsion: 1.7270 Non-1,4 VDW: -0.4999 1,4 VDW: 11.2037 Dipole/Dipole: 0.8558 Total Energy: 48.1659 kcal/mol
13C NMR
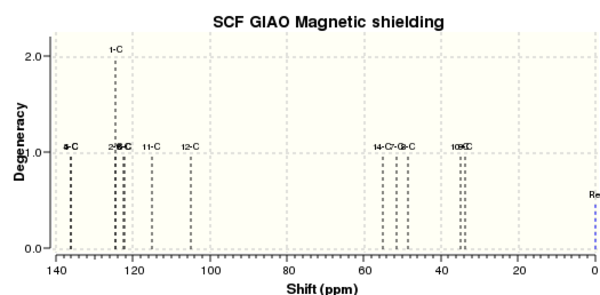
Isomer 1 DOI:10042/22193 DOI:10042/22194
molecule of isomer 1
13C NMR Spectrum of isomer 1
Isomer 2 DOI:10042/22196 DOI:10042/22195
molecule of isomer 2
13C NMR Spectrum of isomer 2
13C NMR were predicted and compared against those in literature. All the calculated values agree well (some even up to 1dp) with the literature values. The difference between the chemical shifts are large enough for the two isomers to be identified. Degenerate orbitals are indicated by the boxes. The 13C NMR spectra have very close resemblance of each other.
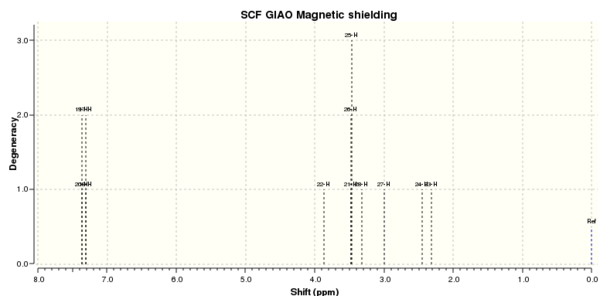
1H NMR
1H NMR is also calculated and compared against literature.
isomer 1 with H labels
1H NMR spectrum of isomer 1
isomer 2 with H labels
1H NMR spectrum of isomer 2
The calculated and literature chemical shifts are summarised below. To find the H atom responsible for the shift, refer back to the two diagrams of the isomers with number labels. Looking at the values of the shifts, the numbers generally have a good match to those from the literature. The boxes indicate degeneracy according to literature (ie for isomer 1, H atoms 18 and 17 are degenerate according to literature as they are in the same box). From literature, H atoms 25, 28 and 27 in isomer 1 are degenerate (all are 3.37). However, according to the calculated shifts, only two of these energies are degenerate (3.356) and the last one is not (3.008). Other examples like this can be found in isomer 2. However the difference is very small and so the computed calculation are still good predictions. Comparing the shifts for the two isomers, all the values are very close together, although there is a small difference, 1H NMR may not be the best technique to differentiate the two isomers.
Optical Rotation
Isomer 1 and 2 are diastereoisomers as the top chiral centre (top of carbon bridge) is common for both. This means that the isomers would give different optical rotation, since they are not mirror images of each other. After performing calculations, it was found that isomer 1 has optical rotation of -48.62 deg whereas isomer 2 has -82.68 deg, nearly double of isomer 1. With such a large difference in value, optical rotation would be one of the key spectroscopic differences of the two isomers.
isomer 1 DOI:10042/22206
isomer 2 DOI:10042/22207
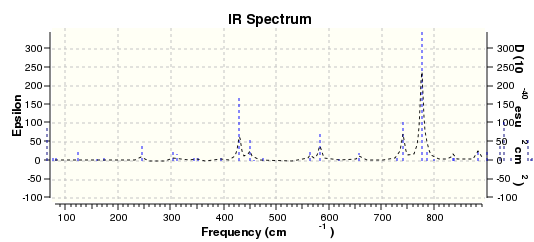
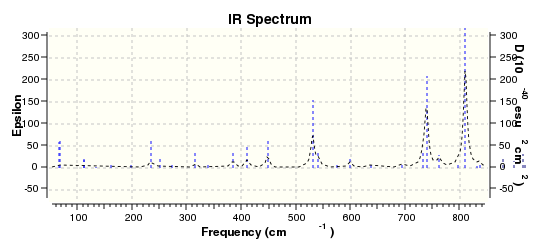
IR
IR calculations were also performed. The energy difference between the two isomers is extremely small. Isomer 1 has 2038828.56 kcal/mol and isomer 2 has 2038830.59 kcal/mol. By inspecting the two IR spectra, you can see that a lot of peaks do not coincide. Three vibrational examples were picked out for comparison. Looking at the two IR tables below for the two isomers, the C-O stretches have different frequencies and intensities. For isomer 1, it has a higher frequency, at 1255.56 Hz, with intensity 52.5002. Whereas for isomer 2 it has a lower frequency, at 1150.32 Hz, with a much higher intensity 176.5924. At frequency of 1508.41 Hz, both isomers have benzene C-H stretches. However the modes are different which can be seen by looking at the animations. Lastly, at around 3117 Hz both isomers have the same kind of benzene asymmetric stretch, with relatively low intensities.
IR spectrum of isomer 1 DOI:10042/22192
IR spectrum of isomer 2 DOI:10042/22191
IR table of Isomer 1:
|
Frequency |
Intensity |
Form of Vibration |
Animation |
|
1255.56 |
52.5002 |
C-O stretch |
|
|
1507.15 |
3.8584 |
Benzene C-H stretch |
|
|
3117.50 |
1.2248 |
Benzene Asymmetric Stretch |
IR table of Isomer 2:
|
Frequency |
Intensity |
Form of Vibration |
Animation |
|
1150.32 |
176.5924 |
C-O stretch |
|
|
1508.41 |
5.6239 |
Benzene C-H stretch |
|
|
3117.93 |
0.6240 |
Benzene Asymmetric Stretch |
Reference
[3] DOI:10.1039/S0040-4039(00)92617-0
[5] DOI:10.1021/j.carres.2007.03.030
[5] DOI:10.1021/jo901398w