Rep:Mod:jr817
BH3
Optimised BH3 molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000037 0.001800 YES RMS Displacement 0.000024 0.001200 YES
Summary Table:
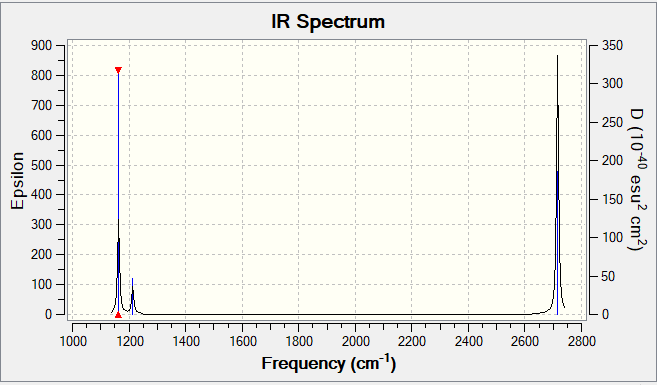
Frequency File: JR817_BH3_FREQ.LOG
Low frequencies --- -3.5991 -1.1355 -0.0055 1.3745 9.7046 9.7707 Low frequencies --- 1162.9825 1213.1733 1213.1760
BH3 vibrational spectrum
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2" | Yes | Out-of-plane bend |
| 1213 | 14 | E' | Slighty | Bend |
| 1213 | 14 | E' | Slighty | Bend |
| 2582 | 0 | A1' | No | Symmetric stretch |
| 2716 | 126 | E' | Yes | Asymmetric stretch |
| 2716 | 126 | E' | Yes | Asymmetric stretch |
There are fewer vibrational peaks in the IR spectrum than there are vibrations as some of the vibrations are degenerate so appear as 1 peak in the IR spectrum (i.e. there are 2 vibrations at both 1213 and 2716 cm-1) and because the vibration at 2582 cm-1 results in no overall change in dipole moment so the vibration is not IR active.
Great table with complete information and thought to the accuracy of the reported values. Good explanation, covering both reasons which lead to only 3 visible peaks. Smf115 (talk) 22:28, 16 May 2019 (BST)
BH3 Molecular Orbital Diagram
There are no significant differences between the real and LCAO MOs. This indicates that qualitative MO theory is very accurate and useful, at-least for highly covalent molecule.
NIce inclusion of the calculated MOs on to the diagram, your comment could have been improved by considering the subtler differences between the real and LCAO MOs though. Smf115 (talk) 22:35, 16 May 2019 (BST)
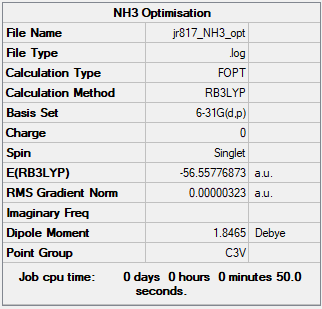
NH3
Optimised NH3 Molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Summary Table:
Frequency File:JR817_NH3_FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
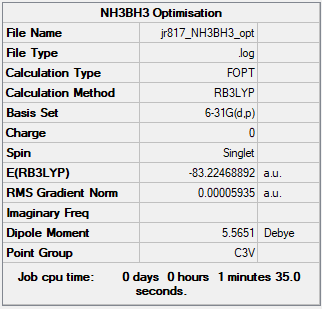
NH3BH3
Optimised NH3BH3 Molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000507 0.001800 YES RMS Displacement 0.000295 0.001200 YES
Summary Table:
Frequency File: JR817_NH3BH3_FREQ.LOG
Low frequencies --- -0.0619 -0.0458 -0.0067 21.6139 21.6198 40.3398 Low frequencies --- 265.9850 632.3698 640.1242
Association Energy of NH3BH3
E(BH3) = -26.61532 a.u.
E(NH3) = -56.55777 a.u.
E(NH3BH3) = -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE= -0.0516 a.u = -135 kJ/mol
This energy calculation shows a negative value for the association energy. This indicates that the formation of the adduct is favourable. As the only physical difference between the 2 reactants and the adduct is the presence of the dative B-N bond. This indicates that the primary contribution is the formation of the dative B-N bond which is relatively weak - compared to a C-C bond (346 kJ/mol).
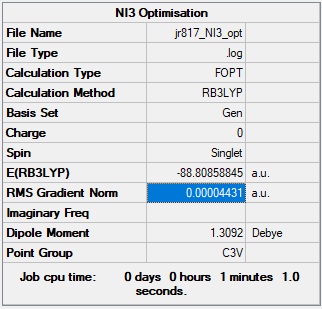
NI3
Optimised NI3 molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000667 0.001800 YES RMS Displacement 0.000490 0.001200 YES
Summary Table:
Frequency file: JR817_NI3_FREQ.LOG
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
The N-I bond distance of the optimised molecule was found to be 2.184 Å.
Correct inclusion of the pseudopotential for the calculation, your structure information is good and clearly presented throughout, the only minor error was using the summary tables from the optimisation and not the frequency calculation. Overall, very good first section though! Smf115 (talk) 22:34, 16 May 2019 (BST)
Ionic Liquids Project
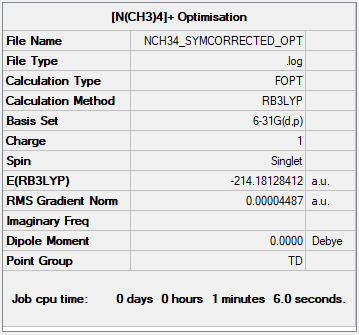
[N(CH3)4]+
Optimised [N(CH3)4]+ molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000151 0.001800 YES RMS Displacement 0.000067 0.001200 YES
Summary Table:
Frequency file: JR817_[N(CH3)4]+_FREQ.LOG
Low frequencies --- 0.0008 0.0009 0.0013 35.2305 35.2305 35.2305 Low frequencies --- 218.8078 317.4871 317.4871
[P(CH3)4]+
Optimised [P(CH3)4]+ molecule |
B3LYP/6-31G(d,p)
Item Table:
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000666 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Summary Table:
Frequency file: JR817_[P(CH3)4]+_FREQ.LOG
Low frequencies --- 0.0013 0.0015 0.0027 51.2698 51.2698 51.2698 Low frequencies --- 186.5950 211.3905 211.3905
Good inclusion of the charges in the calculations and making sure that they are of the correct symmetry. Smf115 (talk) 21:56, 19 May 2019 (BST)
Charge Distribution
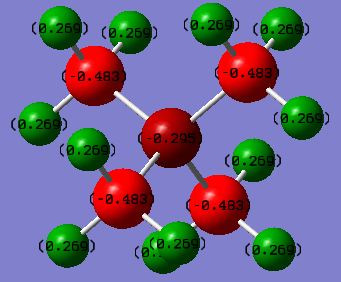
[N(CH3)4]+ Charge distribution:
[N(CH3)4]+ charges:
N = -0.295
C = -0.483
H = 0.269
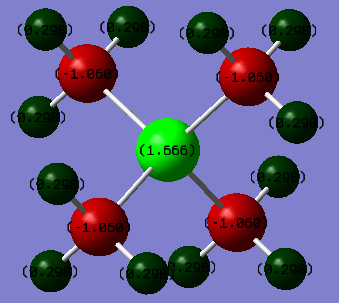
[P(CH3)4]+ Charge distribution:
[P(CH3)4]+ charges:
P = 1.666
C = -1.060
H = 0.298
Here we can see that in P(CH3)4 the positive charge is mostly distributed on the P atoms while the C atoms hold the majority of the negative charge, this contrasts to whats observed in N(CH3)4 where the majority of the positive charge is held on the H atoms and the negative charge is distributed between the N and C atoms, with the C atoms holding more of the negative charge.
The formal positive charge on the N in a traditional picture of N(CH3)4 indicates that the N atom has donated a lone pair of electrons into the electron deficient C of the CH3 group. From the calculations we can see that the positive charge is actually located on the H atoms.
Correct NBO charges calculated, to improve you should have used the same colour range across both molecules to compare the charge distributions. Your discussion sadly lacks any attempt at explaining the relative charge distributions for the two ILs. Additionally, your description of the traditional picture should consider formal electron counting/the Lewis bond picture. Smf115 (talk) 21:55, 19 May 2019 (BST)
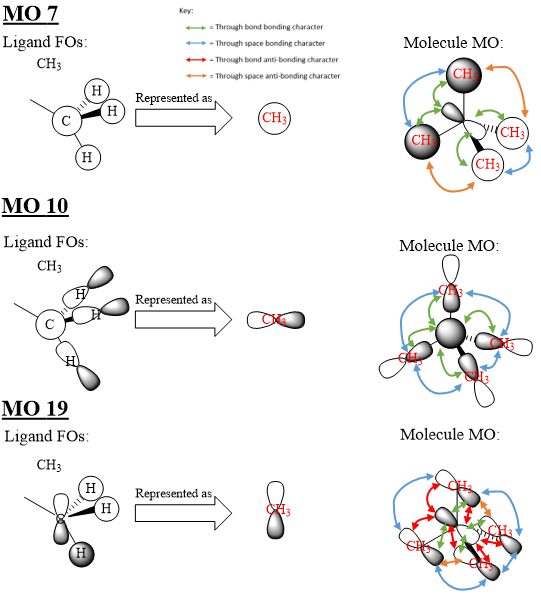
[N(CH3)4]+ Example MOs
There is 1 node in MO 7, 4 nodes in MO 10 and 5 nodes in MO 19. MO 7 and 10 exhibit plenty of strong through-bond and weak through-space bonding character with MO 7 exhibiting little weak through-space anti-bonding character while MO 10 exhibits no significant anti-bonding interactions. Thus, both MO 7 and MO 10 are bonding orbitals. However MO 19, while exhibiting some through bond and through space bonding interactions, also suffers from plenty of strong through-bond anti-bonding character and some through-space anti-bonding character so is an anti-bonding orbital.
Good range of MOs chosen, but where are they! The MOs should have been included in the report to show what your FOs and LCAOs are trying to represent. Good attempt at the FOs but MO10 isn't correct (p orbitals on H?) and you might find it helpful to consider the BH3 MO diagram. To improve your presentation, make sure annotations are large enough to read (the key can't be read), and too many arrows - especially on 19! The symmetry of the molecule can be used, labelling clearly one of these interactions and annotating it on that diagram will make them easier to analyse and read. Smf115 (talk) 21:21, 21 May 2019 (BST)
Overall, a good report with a strong first section in particular. Smf115 (talk) 21:21, 21 May 2019 (BST)