Rep:Mod:jp806physical
Module 3:Physical
Tutorial: The Cope Rearrangement
This section concerns 1,5-hexadiene, there are 27 theoretically possible conformations for 1,5-hexadiene, with 10 of these possibilities existing as distinct structures [1]. A molecule of anti-1,5-hexadiene was first defined in the Gaussview program, the molecule was made inot the anti conformation by setting the central diherdral angle to 180o. The clean structure function was then used to improve the structures intial geometry, before applying an optimisation at the HF/3-21G level of theory. In this project the majority of calculations will be performed using the Hartree-Fock(HF) approximation, this method dates to 1927 and involves approximating the ground state wave-function of a system in terms of a single Slater determinant. Once optimised the anti-strucutre had an energy, E=-231.69262023 au and had a C2 point group, this correlates the anti 1,5-hexadiene defined with "anti1" from the appendix. A second 1,5 hexadiene molecule was then defined this time with a central dihedral angle of 60o to give a conformation that is gauche, this structure was again cleaned and then optimised at the HF/32-1G level of theory to give an energy of E=-231.69266120 au and a C1 point group, this corresponds to the "gauche3" molecule in the table in appendix 1. This result is somewhat surprising since the gauche conformation is of lower energy and one would normally expect the anti strucutre to be the most stable, this more stable gauche conformation in 1,5-hexadiene was also noted by Gung et al. who performed ab initio calculations at the MP2/6-31G* level of theory, there explanation for the stability is an interaction between the CH orbital and the pi-orbital effectively forming CH/pi hydrogen bonds. The formation of these bonds that will stabilise the strucutre is only possible in gauche conformation and therefore the gauche is the more stable conformer. With this in mind and by examining the table given as appendix 1, it can be concluded that the lowest possible energy strucutre of 1,5-hexadiene has already been achieved, images of both conformations can be seen below.
Anti and Gauche 1,5-hexadiene | |||
| Anti 1,5-hexadiene | Gauche 1,5-hexadiene | ||

|
| ||
| E=-231.69262023 | E=-231.69266120 | ||
An attempt was then made to define a molecule with an anti linkage and Ci symmetry in order to do this an anti central carbon backbone was first defined in Gaussview and then then doubly bonded groups added to such an orientation as to match that given in appendix 1, this molecule was then optimised at the HF/3-21G level of theory and the structure had an energy of E=-231.69253528 au, this is the equal to the energy given in the appendix for the Ci conformer to 5 d.p.. It was also found from the calculation summary that the molecule had CI symmetry and it was thus confirmed that the conformation generated was in fact anti2. This structure was the re-optimised at the higher B3LYP/6-31G* level of theory and the energy was re-computed as E=-234.55970436. Although this represents a large change in the energy of the conformer between the two theories there was only a small change in the bond angle (~3.8o) and change in bond length on average of 0.01 Å shorter after the B3LYP/6-31G* level of theory was applied, the geometric data was found by examination of the calculation .log file. Images of the 2 Ci conformers and screen shots of their geometry data can be found below:
Ci Anti 1,5-hexadiene | |||
| HF/3-21G | B3LYP/6-31G* | ||
|
| ||
|
| ||
The structure obtained from the B3LYP/6-31G* theory level calculation was then taken and used in a calculation to determine frequency some thermochemical data and the IR spectrum of the conformer. This was done by selecting the "Frequency" option in the GaussView calculation pane. After the calculation was complete the resulting .log file was first opened in GaussView and the spectrum was checked for imaginery frequencies, none were observed demonstrating that a ground state structure had been found. The thermochemical data was then viewed from the .log file and this data can be found below:
Ci Anti 1,5-hexadiene Thermochemical Data | |||

| |||
These estimates are close to those given for 298.15K in appendix 1, the mean percentage difference between the given and calculated values is 0.011%, with the sum of electronic and thermal energies matching exactly the value given in the table in appendix 1.
Optimising the "Chair" and "Boat" Transition Structure
Firstly an allyl (CH2CHCH2) fragment was defined in GaussView and optimised at the HF/3-21G level of theory, this gave the structure that can be seen below:
Optimised Allyl Fragment | |||
| |||
| E= -304.093 kJmol-1 | |||
This molecule was then copied and pasted into a new GaussView window from there the two molecules were aligned to give a guess of the transition state structure. The terminal atoms on the allyl groups ahve been positioned to be 2.2Å from each other. The image below shows the this intial guess for the transition state that will be used as the input for subsequent chair transition state calculations.
Initial Guess of Chair Transition State | |||
| |||
The intial method used for the identification of the transition state is to carry out an "opt+freq" calculation with the optimisation being carried out to a TS(Berny). The TS Berny option requests that the computer carries out an optimisation to a transition state rather than a global or local minimum, using the Berny optimisation method. Searching for a transition state requires a different process since in a 2D situation a transition state represents a maximum, whereas in a "normal" optimisation one is searching for a minimum. The TS Berny optimisation was then carried out with the calculation of the force constants being carried out once and the Opt=NoEigen, this option allows multiple negative frequencies to be calculated. The results of this calculation at the HF/3-21G level of theory can be seen below:
Transition State Method 1 | |||

| |||
| E= -213.61932245 Hartree | |||
The bond-forming distance in this TS structure has altered from 2.2 Å as in the input to 2.02046 Å and 2.02054 A˚. The vibrational analysis demonstrated that the structure was a transition state (TS) since one negative frequency was observed at -817.963 cm-1, the diagram below shows this negative frequency and indicates that it is the fequency involved in the Cope re-arrangment:
TS Method 1 | |||
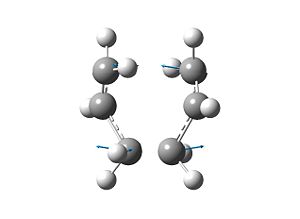
| |||
| Frequency= -817.963 cm-1 | |||
The frozen coordinate method was then applied to the inital guess TS input file for the chair conformer. In this method the Redundant Coordinator Editor was used to freese the co-ordinates of both pairs of terminal allyl carbons, with the distance between them also being fixed at 2.2 Å. A minimisation was then carried out on the input at the HF/3-21G level of theory and yielded the result that can be seen below:
TS Method 2 | |||

| |||
| E= -231.62681760 Hartree | |||
In this case the boding distance between the terminal allyl carbons was 2.2 Å since this is what the distance was set to under the frozen co-ordinate editor. This output was then taken and the Redundant Coordinator Editor was used to change the frozen coordinates, with them being re-defined as Bonds and Derivatives in the option windows, no bond length was fixed. An opt+freq calculation was then run at the HF/3-21G level of theory with a view to optimise to a Berny TS. The results of this calculation can be seen below:
TS Method 2 part 2 | |||
| |||
| E=-231.61835895 Hartree | |||
| Imaginery Frequency= cmsup-1 | |||
In this case the bond-forming distances are 1.95433 &Ating; and 1.90356 Å these are considerably 0.1 Å different from the intial method used, despite using the same level of theory. The QST2 method was then applied to optimise the transition state of the boat conformer, in this method the reactant and product are specified as two unique inputs and the program generates a guess for the TS that is mid-way between the products and reactants, the starting structure is then optimised to a first-order saddle point. In order to apply this method the Ci anti structure of 1,5-hexadiene was used from section 1. This structure was first numbered by visualising the atom labels, the lables were then changed using the atom editor tool and made to match exactly the molecules given in the lab script. The calculation was then carried out at a HF/3-21G level of theory and immediately failed, this maybe because the starting structures are to far removed from the TS. The input was then altered to give the gauche conformer, by changing the central di-hedral angle to 0o and the bond angles of the central carbons to 100o, this gave an input as below:
QST2 Input File | |||
| |||
The calcuation was then run, with opt+freq and the QST2 option selected, at the HF/3-21G level of theory and this gave a boat transition structure with energy (E=-231.60280238 Hartree),a single imaginery frequency at -840.069 cm-1 and a bond forming distance of 2.13996 Å and 2.13976 Å. The imaginery frequency corresponded to the Cope transformation TS and the TS structure corresponded to the boat structure. The images below demonstrate this:
QST2 Analysis | |||
| TS Structure | Imaginery Frequency | ||
|

| ||
A calculation was then run in an attempt to follow the reaction path, this type of calculation is called an Intrinsic Reaction Coordiante (IRC) calculation. In this calculation the input is a pre-determined TS, the calculation then steps in a specified direction towards either the products or reactants by moving along the potential energy surface making small geometric distortions at each step and then optimising the disortions. In this case an IRC calculation was run on both the chair and boat transition state to give an idea as to which structure of 1,5-hexadiene the reaction paths correlate to. The IRC for the chair was set-up with intially 50 steps being employed and following the reaction in the forward direction only, this gave the following results:
IRC Chair | |||
| Step 1 | Step 50 | IRC Trace | |
|
|

| |
This final step was then taken and minimised at the HF/3-21G level of theory, this gave a structure with C2 symmetry and an energy (E=-231.6916691) this suggests that in this case the 1,5-hexadiene conformer of interest is gauche2 as given in appendix 1.An IRC was then run for the boat conformation, this time the calculation was run with 100 steps employed and the initial structure, final structure and IRC trace can be seen below:
IRC Boat | |||
| Initial Structure | Final Structure | IRC Trace | |

|
|
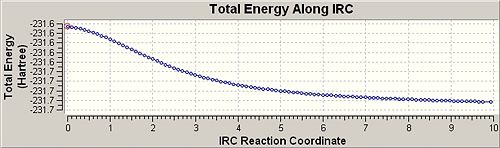
| |
Again the final structure was saved as an input file and optimised at the HF/3-21G level of theory to give a structure with E=-231.6926612 Hartree and symmetry C1. This suggests that the boat conformer forms from the gauche3 model of 1,5-hexadiene. The fact that the chair is formed from a conformer of lower energy than the boat, implies that the chair conformer is more likely to form since its intermediates will be of a lower energy. Both transition structures were then re-optimised at the B3LYP/6-31G* level of theory in order to determine activation energies, as expected a small change in geometry was observed despite a considerable change in energy ~12kcalmol-1 when the two different theory levels were applied. The activation energies were calculated at 298.15K and the results can be seen below:
Activation Energies | |||
| Conformer | Calculated/ kcalmol-1 | Experimental/ kcalmol-1 | |
| Chair | 34.356 @298.15K | 33.5 ± 0.5 @0K | |
| Boat | 41.42 @298.15K | 44.7 ± 2.0 @0K | |
The results show good agreement with the experimentally determined results, this shows high accuracyy in the B3LYP/6-31G* calculation method as well as suggesting that the correct conformers have been identified for the Cope rearrangment reaction.
The Diels-Alder Cycloaddition Reaction
In this section the Diels-ALder reaction will be investigated in terms of MO theory and TS structures. Initially a molecule of cis-butadiene was defined in GaussView and optimised at the HF/3-21G level of theory with the pop=full keyword placed in the additional information entry. The MO's were then visualised using the Edit->MOs option on the GaussView program, the results of this can be seen below:
Cis Butadiene | |||
| HOMO | LUMO | ||
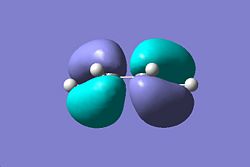
|
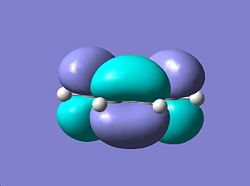
| ||
| Antisymmetric wrt Plane | Antisymmetric wrt Plane | ||
| Symmetric wrt Axis | Antisymmetric wrt Plane | ||
In order for a concerted pericyclic reaction to take place the orbitals must be able to overlap, in this case cis-butadiene is interacting with ethene, since ethenes LUMO has the same symmetry properties as cis-butadiene's HOMO it can be concluded that these two pairs of orbitals overlap, it is for this reason that the endo product is favoured. The transition structure was then calculated at the HF/3-21G level of theory using the Berny TS in the optimisation. The initial bond forming distances were defined as 2.2 Å and the calculation was sucessful. The optimised TS structure had an energy E=-231.60320844 Hartree and bond forming distances of 2.2102 Å and 2.20854 Å and the TS was confirmed by an imaginery frequency at -818.777 cm-1, that correlates to the Diels-Alder. The HOMO orbital of the TS structrue was then visualised from the check point file and this can be seen below:
HOMO Cis-butadiene TS | |||
| HOMO | |||
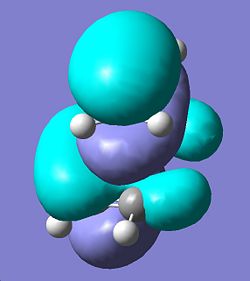
| |||
| Antisymmetric wrt Plane | |||
| Antisymmetric wrt Plane | |||
The regioselectivity of the Diels-Alder reaction was then investigated using the exo and endo forms of a reaction between Cyclohexa-1,3-diene and maleic anhydride. Firstly the exo-transition state was investigated this was deduced by running an opt+freq calculation to a TS Berny at the HF/3-21G level of theory. Initially the bond forming distance was set to be 2.2Å apart this calculation failed and so the bond forming distance was re-set to be 2.25 Å this ran a successful calculation and generated the TS that can be seen below, the energy of this TS was E=-=-605.60359125 Hartree and there was one imaginery frequency at -647.464 cm-1 confirming the presence of a TS. The final bond forming distances were found to be 2.26069 Å and 2.26076 Å, the HOMO was generated from the .chk file and this can also be seen below.
Exo | |||
| TS | HOMO | ||
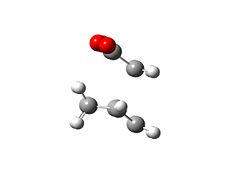
|
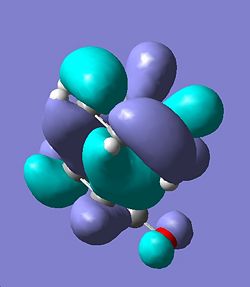
| ||
The endo form of the product was then investigated by determining the Berny TS at the HF/3-21G level of theory, this was slightly more probelmatic than finding the TS for the exo form however with the bonding distances set to 2.2Å. After optimisation the TS had energy E=-605.61036809au, with one imaginery frequency at -643.604 cm-1 that can be confirmed as the TS for the diels-alder reaction. The bond forming distances were calculated as 2.23118Å and 2.23079 Å. The HOMO was then plotted from the .chk file and this can be seen below:
Endo | |||
| TS | HOMO | ||
| File:Jp806endots.jpg | File:Jp806endohomo.jpg | ||
From these results it can be seen that the endo form has a lower energy TS this suggests that the reaction proceeds via kinetic control with the endo product being primarily formed. The molecular orbitals of the TS suggest stronger σ-bonding in the exo form this is also demonstrated by the shorter length of one of the bond forming distances in the the exo case. The fact that the endo case is more stable as a product shows that the MOs seen in the TS are not fully formed MOs.
- ↑ Gung B.W, 1995, J. Am. Chem. Soc, 1176(6), 1783-1788 DOI:10.1021/ja00111a016
