Ammonia Inversion
| Inversion Mechanism Potential Energy Surface
|

|
The Vibrations of Ammonia
The frequency calculations for C3v and D3h ammonia were computed using the B3LYP method with a 6-31G basis set. The results of these calculations can be seen in the table below.
Ammonia Vibrations
|
| Vibration
|
C3v Frequency/cm-1
|
C3h
|
D3h Frequency/cm-1
|
D3h
|
| 1
|
1357.44
|

|
(-840.58)
|
|
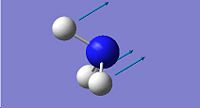
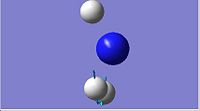
| 2
|
1937.93
|
|
1587.95
|
|
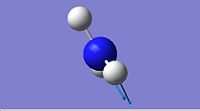
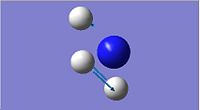
| 3
|
1937.93
|
|
1587.95
|
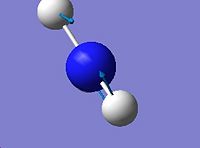
|
| 4
|
3393.56
|
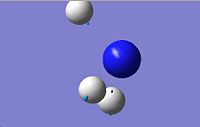
|
3676.66
|
|
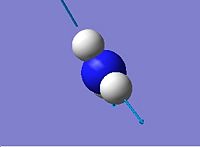
| 5
|
3659.31
|
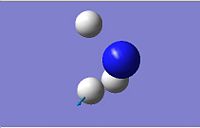
|
3894.23
|
|
| 6
|
3659.31
|
|
3894.23
|
|
Both the D3h and C3v symmetries have 6 possible vibrational modes with 4 vibrational frequencies although in the case of the D3h symmetry there are only 3 peaks that would appear in a real spectrum since a negative frequency is not physically possible. The frequencies labelled 1 in both cases could be used to follow the course of the inversion. Since they are unique frequencies for each symmetry. The table below compares the values obtained from this computation with those obtained experimentally for the ground state molecule of ammonia.
Ammonia Vibrations Compared with Literature [1]
|
| Vibration
|
Calculated Frequency/cm-1
|
Observed Frequency/ cm-1
|
| 1
|
1357.44
|
932
|
| 2
|
1937.93
|
1626
|
| 3
|
3393.56
|
3337
|
The computed results show a good correlation with the experimental results showing the high level of accuracy in this calculation. The difference is particularly small at lower wavelengths.
TM Complex
The transition metal complex Mo(CO)4(PMe3)2 was first defined on GaussView and the optimised using the B3LYP protocol with the LANL2MB basis set. In order to ensure convergence the opt=loose option was added to the calculation. This calculation was then submitted to the SCAN server for optimisation.
TM Complex Optimisation 1
|
| Cis
|
Trans
|
Cyclopentadiene Endo Dimer | | |
|
Cyclopentadiene Endo Dimer | | |
|
| Total Energy=
|
Total Energy=
|
The .log files were then saved as .gjf files and the complexes re-optimised using the same method but with a LANL2DZ basis set to improve accuracy. The molecules were uploaded to scan with the additional information int=ultrafine and scf=conver=9 input. The results of this improved optimisation can be seen below.
TM Complex Optimisation 2
|
| Cis
|
Trans
|
Cyclopentadiene Endo Dimer | | |
|
Cyclopentadiene Endo Dimer | | |
|
| Total Energy=
|
Total Energy=
|
The IR spectras for both isomers were then computed using the B3LYP method and the LANL2DZ basis set, the resulting spectra can be found below:
TM Complex Optimisation 1
|
| Cis
|
Trans
|

|

|
The spectra show that the trans complex has fewer carbonyl stretches, this is due to the pseudo symmetry of the complexes. The cis isomer is C2v and the trans isomer is D4h, in the D4h point group not all stretches are active in the IR spectra as there is no change in dipole moment. The cis isomer should give 4 CO stretching frequencies whereas the trans isomer should give 3 CO stretching frequencies [2]. The results of the calculation also show the trans isomer to be the most stable as expected. The values obtained for the IR CO stretching frequencies were compared with the literature, this comparisson can be seen in the table below.
IR Literature Comparisson [3]
|
| Literature Cis/cm-1
|
Calculated Cis/cm-1
|
Literature Trans/cm-1
|
Calculated Trans/cm-1
|
| 2012.6
|
2464.8
|
2050.4
|
1882.88
|
| 1912.5
|
2381.58
|
1933.9
|
1839.42
|
| 1899.5
|
2369.85
|
1886.1
|
1839.42
|
| 1886.8
|
2352.29
|
*******
|
*******
|
These values show poor correlation between experimentally observed and calculated values, however the ligands were different in the TM complex. This means that not all of the error can be attributed to error in calculation. In order to improve these calculations an additional basis set was added to the calculation. This allowed for the hydpervalence nature of P. This calcualtion was then submitted to scan for minimisation. Unfortunately only the cis-isomer minimised succesfully and this can be seen in the table below. If time had allowed a second IR spectrum could be generated for the isomers and this could be compared to literature. One would expect these values to be closer to the experimentally obtained values.
Final Optimisation of Cis Isomer
|
Cyclopentadiene Endo Dimer | | |
|
Mini-Project
An intial idea for this mini-project was an investigation into the Belousov-Zhabotinsky reaction that is used in chemical image processors along with a Ru complex ion. This project was deemed to complex for this project but an interest in Ru complexes was sparked. Due to the complex used in the image processor there was no scope for a mini-project as no geometric isomers came to mind immediately. Instead a Ru complex was selected from the literature [4] and the cis and trans isomers investigated. The hope was that after investigation of these isomers optimal structure a rationalisation could be formed for the observed behaviour in terms of molecular orbital theory. The literature [5] demonstrated that the trans isomer was the most stable form of the complex and this would give a grounding for comparisson with literature. An extension to the project could be to investigate the conversion from the trans to the cis isomer than can occur in the presence of dichloromethane and light.
Initially both molecules were defined using the GaussView GUI, these can be found below:
Input Molecules for Mini-Project
|
Cyclopentadiene Endo Dimer | | |
|
Cyclopentadiene Endo Dimer | | |
|
These molecules were then minimised intitally using the B3LYP method and the LANL2DZ basis set, the optimisations were upload onto the SCAN server for processing and from this point the problems began. The trans isomer was returned from SCAN and opened on the GaussView GUI unfortunately later evaluation of the .log file from the output highlighted an error "The combination of multiplicity 1 and 505 electrons is impossible". The cis isomer had ran some 15 itterations before the calculation was terminated due to the time constraints of the project the conditions of the last optimisation can be seen below and it is clear that the calculation had failed to reach the Gaussian convergence criteria outlined in section 1 of this report. The Gaussian output can be seen below for the final stage in the aborted optimisation procedure.
Gaussian Output
|

|
These errors could be avoided if the project was to be run again, the time out of the cis optimisation could be avoided by reducing the level of the basis set used to for example LANL2MB, then optimising in a subsequent step with the 2DZ method. Furthermore the maximum number of cycles could be reduced in order to ensure an optimisation. If the project had been successful a more advanced calculation could be run giving an extra basis set to the P atoms to allow for the possibility of their hypervalence. This would sucessfully optimise the complex and hence allow further calculations to be run to determine the MO of the complex. In some respects the error seen for the trans isomer is more worrying, this means that there are 505 electrons in the system since since multiplicity is set to 1 (implying all electrons are paired) this is clearly impossible and Gaussian detected this during its intial book keeping on the molecule and terminated the job. This problem could be resolved by either re-defining the molecule to ensure that the electron count is correct or by setting the charge multiplicity equal to any even number. If the charge multiplicity was set equal to 2 for example then the lowest energy doublet state of the molecule would be determined.
The MO calculation for the trans complex was also attempted but this subsequently failed, this is because the trans complex had not succesfully optimised on the SCAN server and this was not realised until subsequent analysis of the .log file in its text format.
Evaluation of Methods Used
Density Functional Theory[6]
The model used for the initial part of this wiki was the that of DFT this is an established quantum mechanical model first proposed in the 1970's. This model relies on the Born-Oppenheimer approximation that is that the position of the nuclei are fixed from the point of view of the electron (since the nucleus is so large any movement of the nucleus will lead to an instantaneous movement of electrons) and is an extension of the Hartree-Fock approximation. In DFT electrons are model via "general functionals" [6], where a functional is a function of another fucntion. Current DFT models compose of 4 terms into which the energy of moelcule can be decomposed, these are a kinetic energy term, a nuclear-electron interaction term , an electron-electron repulsion term and an exchange correlation term, . The last term can be further divided into a correlation and exchange term, and as with all terms except nuclear-nuclear interactions determined from electron density calculations. All terms bar the exchange-correlation term can be thought of in terms of classical physics. The difficulty in calculating a DFT is determing the exchange-correlation term, this is only known for the free electron gas but can be approxiamted by integration. Gaussian approximates the exchange correlation term via numerical integration usually through a hybrid functional (a hydrib functional involves the use of Hartree-Fock and other (model dependant) functionals). In this project DFT calculations have been run using the B3LYP method this means Gaussian is solving the hybrid functional shown below in order to determine the exchange-correlation energy:

This may seem to be a relatively simply formula but when just a few of the terms are expanded one is very glad that the computer is capable of numerically solving such functions!
Moller-Plesset Pertubation Theory[6]
This theory relies on the mathematical concept of a pertubation, this is when an approximate solution to an equation is determined by knowing an exact solution to a similar problem and building fromt that exact solution a power series to describe approximate solution. In this case the Hamiltonian is approxiamted by breaking the total Hamiltonian into two components a known Hamiltonian and term for pertubation. The exact method of pertubation theory in Gaussian and the mathematical meaning of pertubation theory cannot be explored here due to time constraints. It is worth noting that MP2 theory does include a more accurate exchange-correlation term than DFT.
- ↑ D.Dixon, J. Phys Chem. A, 2005, 109, 5129-5135 DOI:S1089-5639(04)04562-1 10.1021/jp0445627 S1089-5639(04)04562-1
- ↑ Darensbourg, Inorg. Chem, 18, 1, 1979
- ↑ Darensbourg, Inorg. Chem, 18, 1, 1979
- ↑ Diez, Gamase et al, Eur. J. Inorg. Chem., 2004, 10, 2078-2085
- ↑ Diez, Gamase et al, Eur. J. Inorg. Chem., 2004, 10, 2078-2085
- ↑ 6.0 6.1 6.2 Foresman & Frisch, Exploring Chemistry with Electronic Strucuture Methods, Gaussian Inc, Seconfd Edition, 1993 Cite error: Invalid
<ref> tag; name "usersguide" defined multiple times with different content
















