Rep:Mod:jotp1357
Cope Rearrangement
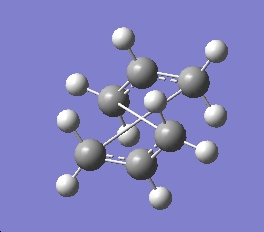
anti-hexadiene
anti2 HF/3-21G energy= -231.69253506
point group= Ci with sub group C1
Pentahelicene |
gauche-hexadiene
energy= - 231.69266120 point group= C1 with subgroup C1 aswell.
reoptimised antihexadiene
experimental values
Sum of electronic and zero-point Energies= -233.070012 Sum of electronic and thermal Energies= -233.062608 Sum of electronic and thermal Enthalpies= -233.061664 Sum of electronic and thermal Free Energies= -233.101686
After reoptimising the anti2 linkage of hexadiene the energy lowered using the B3LYP/6-31G*. The geometry only changed very slightly, as the bond angles changed from 111.36 to 112.41 for C-C-C and from 124.80 to 124.91 for C=C-C.
Chair and Boat Transition Structures
Chair
the vibration corresponding to the cope rearrangement was at -817.952cm-1 with an intensity of 5.9611.
bond forming= 1.5501 bond breaking= 4.74518
I found if the fragments were positioned more than 2.2 apart eachother then i had problems with running the calcualtion for the transition state. Once i had the bond lengths just lower than 2.2 then the calculation ran smoothly.
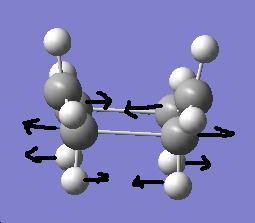
After doing the calculation by freezing coordinates method at the fixed bond lengths of 2.2 the chair transition state looked the same as above. Once the bonds were not fixed, the transition state looked changed, looking like the vibration that occurs in the bond breaking/forming. The transition state is showned below.
Boat
Initially the job failed when doing the TS (QST2) until the bond angles and dihedral angles of the reactant and product when changed as the image below shows.
the transition state:
the transition state frequency at -839.497cm-1 at 1.607 intensity
Results Table
Summary of energies (in hartree)
| HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | B3LYP/6-31G* | |
| Electronic energy | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | |||
| Reactant (anti2) | -231.6925 | -233.2099 | -233.0700 | -233.1017 |
Diels-Alder Cycloaddition
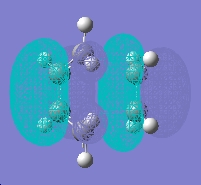
The molecular orbital is antisymmetric respect to the plane and the axis.
The molecular orbital is antisymmetric respect to the plane nut symmetric respect to the axis.
When the cisbutadiene reacts with the alkene the symmetry properties of the orbitals that are overlapping need to be the same therefore the LUMO of ethene needs to antisymmetric to the plane and symmetric to the axis. this will keep the the stereochemistry of substituents.
The transtion state frequency is at -818.548cm-1. The value suggests it a certain concerted pericyclic cycloadiition reaction. The next frequency is 166.607cm-1 this corresponds to the the rocking of the ethene fragment.
The fragments were set to be 2.2 angstroms for eachother.
Normal values for C-C bond lengths:
Double bond=1.34Å and single bond=1.54Å
My values have the double bonds at 1.37Å and the single bonds at 1.40Å throught the transition state.
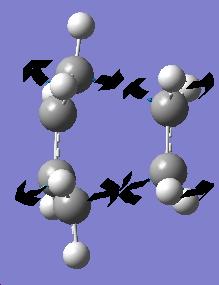
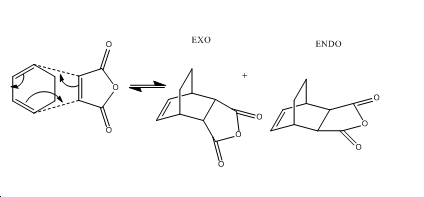
The Regioselectivity of the Diels Alder Reaction
Endo
optimised energy= -609.403a.u.
Exo
optimised energy= -609.363a.u.
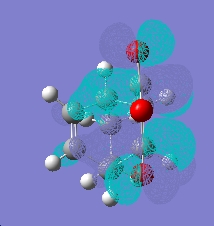
When looking the HOMO orbitals of the two products they are very different. If looking at the -C=O-CO-C=O- fragment compared to the rest of the molecule, the electron density moves depending on the product. Most of the electron density seems to sit on the part of the -C=O-CO-C=O- fragment closest to the CH=CH fragment of the ring in the exo product. This seems to be a favourable interaction as the phases of the orbitals are the same. But the endo product the orbitals has an unfavourable interaction with the CH2-CH2 fragment.
References
1. http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html