Rep:Mod:joto1357
The Hydrogenation of Cyclopentadiene dimer
Dimer 1
Pentahelicene |
Stretch: 1.2923 Bend: 20.5870 Stretch-Bend: -0.8413 Torsion: 7.6715 Non-1,4 VDW: -1.4358 1,4 VDW: 4.2320 Dipole/Dipole: 0.3778 Total Energy: 31.8834 kcal/mol
Dimer 2
Stretch: 1.2454 Bend: 20.8603 Stretch-Bend: -0.8320 Torsion: 9.5039 Non-1,4 VDW: -1.5083 1,4 VDW: 4.3012 Dipole/Dipole: 0.4448 Total Energy: 34.0153 kcal/mol
The stability of dimer two is higher in energy than the dimer one. Therefore you would expect dimer one to be the most stable conformation if the products were thermodynamically controlled. This suggests that dimer one is less strained and hindered thermodynamically compared the starting material cyclopentadiene. But this isn’t seen as molecules three and four are produced from the hydrogenation of dimer two. Therefore the stability of this cyclopentadiene dimer is determined by kinetic control (the stability of the transition state.) The transition state of dimer two must have lower ∆G # therefore the amount of energy required to overcome the energy gap is less.
hydrogenation of dimer 2 gives the two following prodcuts
Molecule 3
Pentahelicene |
Stretch: 1.2136 Bend: 18.8637 Stretch-Bend: -0.7566 Torsion: 12.2394 Non-1,4 VDW: -1.5531 1,4 VDW: 5.7620 Dipole/Dipole: 0.1631 Total Energy: 35.9321 kcal/mol
Molecule 4
Stretch: 1.2067 Bend: 18.8637 Stretch-Bend: -0.7528 Torsion: 12.2396 Non-1,4 VDW: -1.5532 1,4 VDW: 5.7649 Dipole/Dipole: 0.1632 Total Energy: 35.9322 kcal/mol
If looking at the thermodynamic properties of molecules three and four are considered than the more stable product would be molecule 3. Which corresponds to the relative H bonding in the two structures as the dipole/dipole interaction in molecule 3 is higher than four therefore more stability in 3. Also the bending energy in 3 is higher suggesting more flexibility in this product, as larger dipole interaction. When comparing the torsion between the two molecules molecule 4 has a lower value, this corresponds to the bending values of the molecules are it is more strain.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
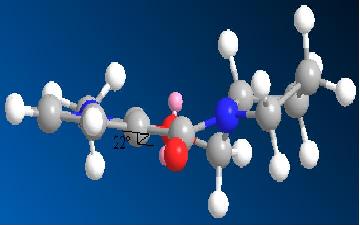
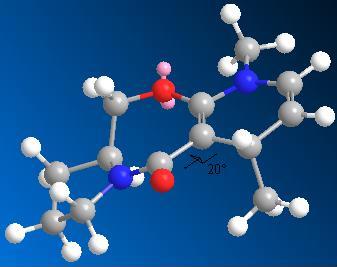
Molecule: 5+6
Pentahelicene |
Stretch: 1.1760 Bend: 11.3848 Stretch-Bend: 0.0549 Torsion: 5.1335 Non-1,4 VDW: -1.9345 1,4 VDW: 11.8795 Charge/Dipole: 2.6813 Dipole/Dipole: -3.9695 Total Energy: 26.4060 kcal/mol
The dihedral angle is 22 between the carbonyl and the benzene ring which you wouldn’t expect. This is because of the addition of the Grignard.
Stretch: 1.4194 Bend: 14.3371 Stretch-Bend: 0.1616 Torsion: 5.4551 Non-1,4 VDW: -2.2656 1,4 VDW: 13.1728 Dipole/Dipole: -3.9970 Total Energy: 28.2835 kcal/mol
Its still not flat between the carbonyl an d the ring. The methyl group attaches in out of the plane geometry, the opposite face to the carbonyl of this bent geometry.
The ratio of molecule 6 to the addition of the methyl group onto the pyridine group is 3:1 whish retains the stereochemistry. Increasing the bulkiness of the group (EG phenyl groups ) increases the selectivity increasing the amount of molecule made.
The addition of MeMgI to molecule 5, the coordination stays on the face of the oxygen therefore the carbonyl group is bend away from the pyridine the coordination is also bent away. Therefore when the methyl anion attacks the ring it does this from the opposite face because of the obvious steric factors involved. The main stereochemistry of the addition on the methyl group comes from the intramolecular addition through a six centred transition state.
Molecule 7+8
Pentahelicene |
Stretch: 1.5944 Bend: 6.8488 Stretch-Bend: 0.3267 Torsion: -6.0614 Non-1,4 VDW: -1.7470 1,4 VDW: 17.5552 Charge/Dipole: 2.3415 Dipole/Dipole: -4.8044 Total Energy: 16.0537 kcal/mol
Stretch: 1.4698 Bend: 16.3493 Stretch-Bend: 0.4189 Torsion: -29.6558 Non-1,4 VDW: -7.3365 1,4 VDW: 14.8382 Dipole/Dipole: -5.0289 Total Energy: -8.9452 kcal/mol
There stereo chemistry stays as explained above for molecule explain this product ratio is higher as the reagent has a phenyl ring which is obviously bigger than a methyl this favours this reaction.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Stretch: 2.5517 Bend: 10.6988 Stretch-Bend: 0.3241 Torsion: 19.5766 Non-1,4 VDW: -1.2275 1,4 VDW: 12.5515 Dipole/Dipole: -0.1826 Total Energy: 44.2927 kcal/mol
Pentahelicene |
Stretch: 2.5548 Bend: 10.6788 Stretch-Bend: 0.3276 Torsion: 19.5651 Non-1,4 VDW: -1.1572 1,4 VDW: 12.5567 Dipole/Dipole: -0.1825 Total Energy: 44.3434 kcal/mol
Both structures have the six membered ring in the chair conformation therefore the stability of the two are similar, one is stable due the dihedral angle between the ring and the carbonyl group. Molecule 10 is lower in energy therefore more stable as there is less steric strain between the carbonyl group and the ring as the dihedral angle is 78° compared to structure 11 and the dihedral angle is 77°. The two conformers are atropisomers. This means rotation about the single bonds is hindered due to steric strain therefore hard isolate them.
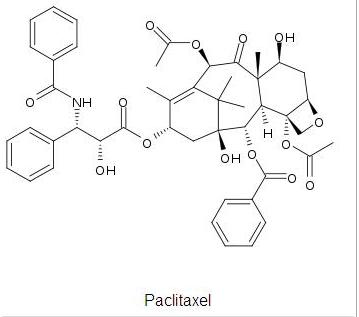
The image above shows taxol which is the desired product after the intermediate state (molecule 10 and 11). One of these intermediate continues on in the reaction to produce taxol which one depends on the orientation of the carbonyl group. Molecule 10 is the key intermediate for the production of taxol as the orientation of the carbonyls corresponds as it allows addition groups to attach to the molecule from the opposite face.
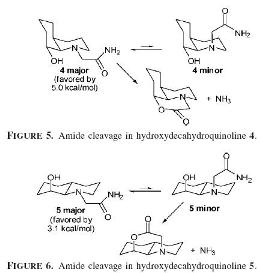
Hydrolysis of a peptide
Stretch: 1.6725 Bend: 3.6988 Stretch-Bend: 0.5251 Torsion: 7.6517 Non-1,4 VDW: -3.3047 1,4 VDW: 9.6400 Dipole/Dipole: -3.2239 Total Energy: 16.6595 kcal/mol
Stretch: 1.5322 Bend: 3.2493 Stretch-Bend: 0.4853 Torsion: 7.2288 Non-1,4 VDW: -8.1380 1,4 VDW: 9.7778 Dipole/Dipole: -5.0650 Total Energy: 9.0704 kcal/mol
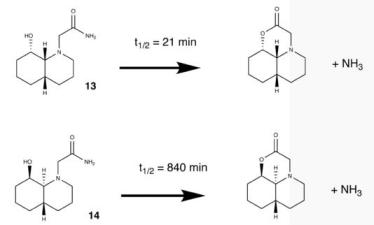
As the reaction above shows the half life is shorter for the cis decalin conformation as the amide group in the same plane as the hydroxyl to allow the reaction to occur. When you optimised molecule 13 and 14 you get the geometry suggested as the major reactant in the reaction schemes above show. Seeing as the equilibrium lies on the minor conformation of the equilibrium for molecule 14 where the hyrdoxyl group is axial. As it is a minor reactant the half time is much time as the molecule prefers to lie on the other side of the equilibrium.
Hydrogen bonding is the first conformation compared to the second is less due the fact that the carbonyl and hydroxyl groups are very close to each enough. Therefore this would suggest the second is more stable which slows down the hydrolysis reaction. Kinetically the first is much faster even though thermodynamically it is more stable as both the functional groups in the major conformation in the equilibrium are equatorial. This differs from the major conformation of the second as one group is axial and equatorial decreasing the stability energy.
Regioselective Addition of Dichlorocarbene
Stretch: 0.6113 Bend: 4.7976 Stretch-Bend: 0.0410 Torsion: 7.6228 Non-1,4 VDW: -1.0741 1,4 VDW: 5.7882 Dipole/Dipole: 0.1119 Total Energy: 17.8987 kcal/mol
When deciding where electrophilic addition would occur on the dichlorocarbene, it depends on the stability of each alkene. The exo pi orbital corresponds to the HOMO-1 orbital, the endo pi orbital corresponds to the HOMO orbital and the Cl-C sigma* orbital corresponds to the LUMO+2 orbital. The HOMO-1 is more delocalised therefore more stabilised interaction with the Cl-C sigma* orbital leaving the endo pi orbital more nucleophilic, susceptible to nucleophilic addition if looking at the stereoelectronics. Stereoelectronics normally outweighs the regiochemistry as the literature would allow electrophilic addition at the exo pi bond.
The dichlorocarbene has Cs symmetry but its monohydrated derivative doesn’t, it has lost a bit of symmetry therefore less stable and rigid. This corresponds to the slight increase of absorption in the stretching of the c=c bond in the monohydrated product. The vibration of the C-Cl bond is very weak.
Mini Project
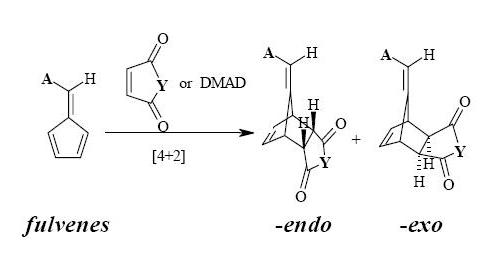
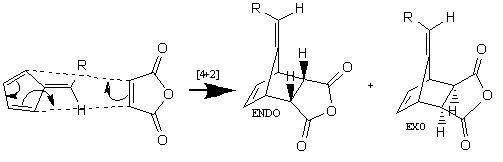
In this project i endeavour to distinguish between the endo and exo products of the reaction scheme below. The reaction is a Diels-Alder cycloaddition (4+2) of N-phenylmaleimide and maleic anhydride, with the following mechanism. The products are separated using flash chromatography. The endo adduct is the major product, therefore throughout this investigation i intended to find out why the endo product dominates using spectroscopic analysis. Note: the R group is 3.4-(OCH3)2C6H4
Eno
Pentahelicene |
NMR http://hdl.handle.net/10042/to-1233 IR http://hdl.handle.net/10042/to-1235
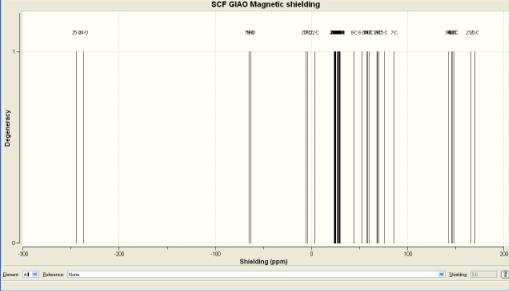
-2.34114 and -3.80435 carbonyl carbons on the R group. 27.9117 and 27.7833 are the chemical shifts due to the alpha hydrogens coupling with the carbons on the anhydride ring.
The ring junction carbon at 39.3805.
Bridgehead carbons at 63.2743 and 61.2832 chemical shifts. 163.629 is the chemical shift due to the carbons in the methyl adjacent to the ketone carbonyls.
Exo
Pentahelicene |
NMR http://hdl.handle.net/10042/to-1232 IR http://hdl.handle.net/10042/to-1234
-5.62268 and 3.20632. 28.3358 and 28.3416 are the chemical shifts due to the alpha hydrogens coupling with the carbons on the anhydride ring. The ring junction carbon at 44.4537. Bridgehead carbons at 59.8832 and 58.8407 chemical shifts. 165.805 and 169.558 are the chemical shifts due to the carbons in the methyl adjacent to the ketone carbonyls.
13C NMR
The literature ran the NMR samples using CDCl3 as the solvent in a 400 MHz spectrometer.
The literature suggests chemical shifts of the endo adduct at 170 for the carbonyl groups. 49 and 54 for the bridgehead carbons and 45 and 46 for the ring junction carbons.
The main differences between the NMrs of the products are the degeneracy of the coupling protons and the chemical shifts of the carbonyls of the ketones. The degeneracy of the protons on the carbonyls in ketone goes up to 4 on the endo product, as two of the protons in on the methyl are in the same environment but in the exo product the degeneracy only goes to one the protons are mostly all in different environments. The carbons in the same carbonyls are quite separated in their chemical shifts in the exo product compared to the endo (chemical shifts very close). These two products allow you to distinguish between the two product
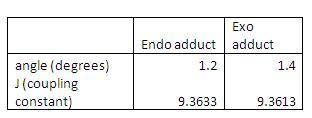
3JH-H coupling To distinguish between the two products it is best to look at the coupling of the alpha protons on the products. As the alpha protons interaction differ in the endo and exo products due to their different orientation. The table below shows the coupling constants:
As you can see from the table above there is a slight difference in the figures. This probably due to the fact that the splitting of the alpha protons in a H NMR would be a doublet in the exo product and a singlet in exo product ( as suggested in the literature.
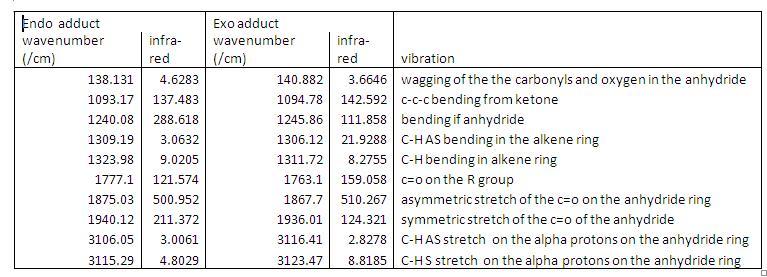
IR spectra The table below shows the main IR absorptions:
The obvious main IR absorption in the literature is at 1750cm-1 signifies the carbonyl stretch. From the spectrum the carbonyl shifts of the two products are a little higher than 1750cm-1 for the carbonyls on the group and much higher of the anhydride ones (~1880cm-1). When trying to distinguish between the exo and exo adducts when looking at the IR spectrums there is a big difference in the strength of the absorption of the asymmetric stretch of the C-H bonds in the alkene ring. The exo product has a much stronger peak. Also the alpha protons on the anhydride ring symmetric and asymmetric stretches are out by 10cm-1 between the endo and exo products.
References
1. SEPARATIONS AND CHARACTERIZATIONS OF CONFIGURATIONAL STEREOISOMERS FROM CYCLOADDITION REACTIONS OF NEW METHOXY SUBSTITUTED-6-ARYLFULVENES http://aacd2004.adu.edu.tr/abstracts/short-pdf/300.pdf
2. M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
3. B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
4. Structure of Taxol http://en.wikipedia.org/wiki/Paclitaxel