Rep:Mod:joti1357
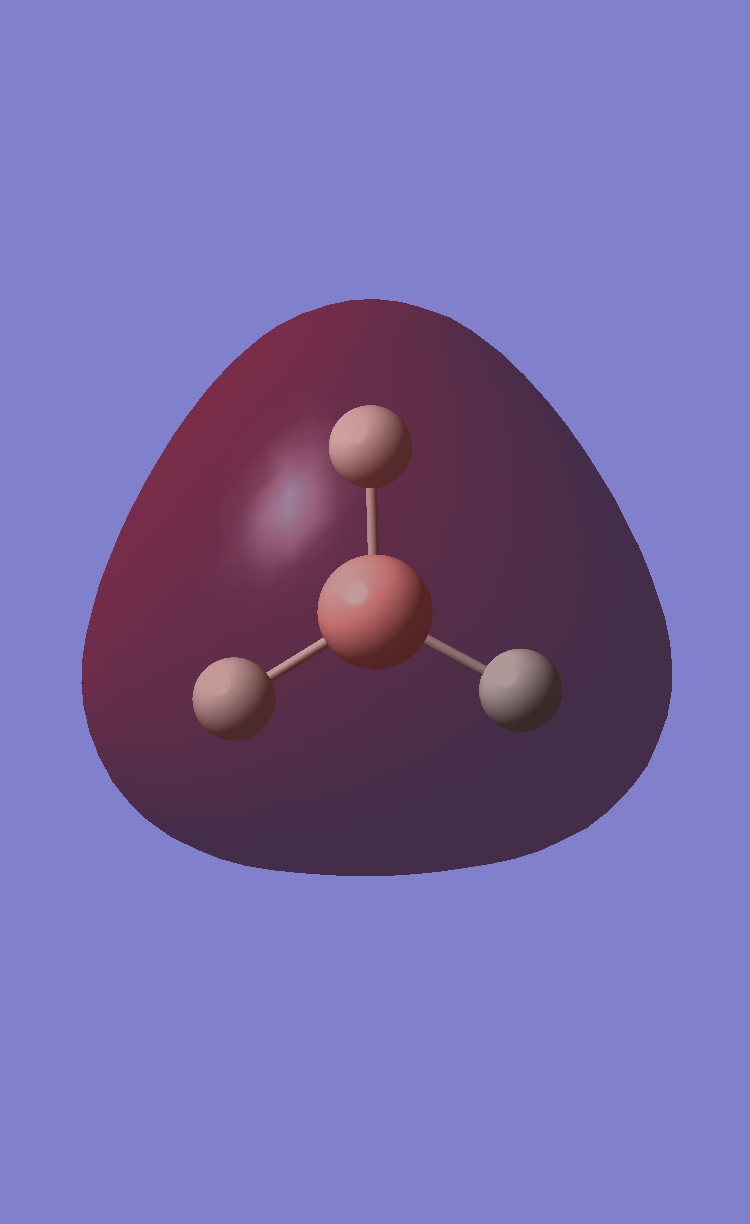
BCl3
The optimised B-Cl bond length=1.86592 The optimised Cl-B-Cl angle=120.00 File Type: .log Calculation Type: FOPT Calculation Method: RB3LYP Basis Set: LANL2MB Final Energy: -69.43928112 Dipole Moment: 0.000D Point Group: D3H Calculation Time: 18s
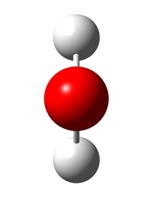
H20
The optimised H-O bond length=0.9968 The optimised H-O-H angle=103.9905 File Type: .log Calculation Type: FOPT Calculation Method: RB3LYP Basis Set: 3-21G Final Energy: -75.97396515 Dipole Moment: 2.2381D Point Group: C2V
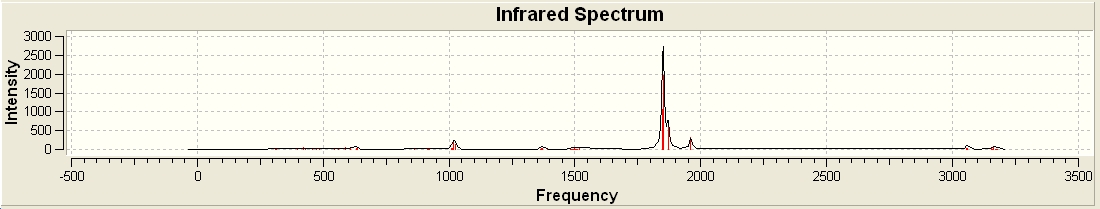
vibrations
Although there are six vibrations there are only three peaks on the IR spectrum, as there are two of the vibrations are degenerate, a stretch and a bend. Also the symmetric stretch doesn’t have an infra-red value as the isn’t a change in the dipole moment when that vibration occurs.
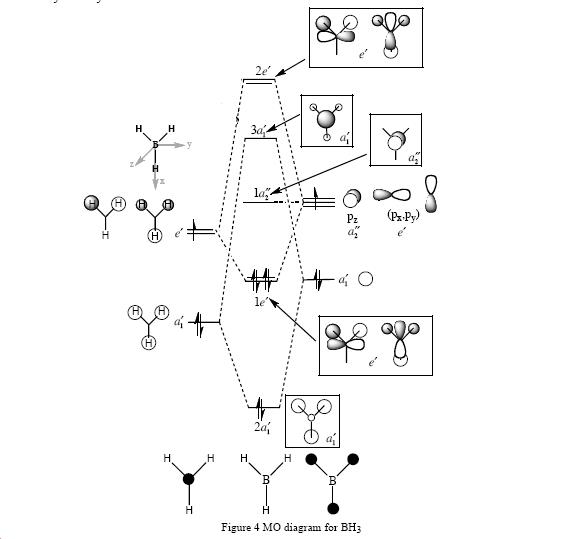
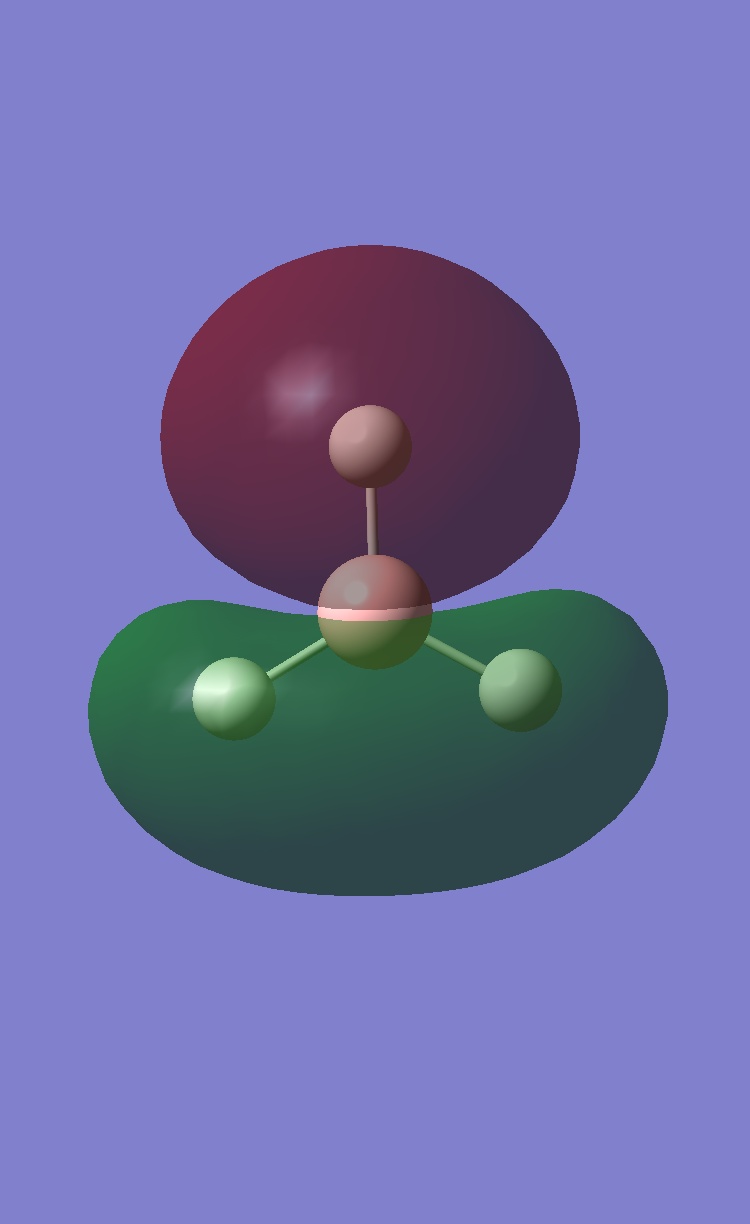
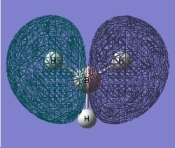
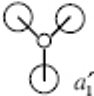
predicted qualitative MOs
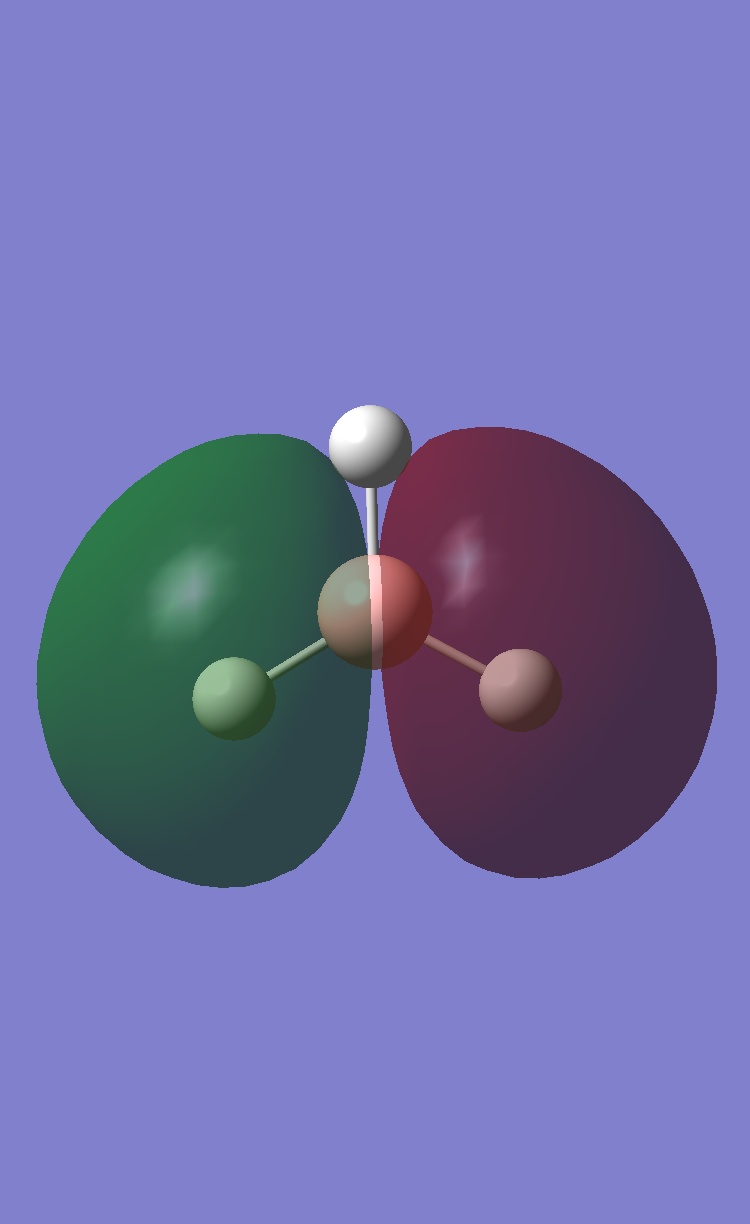
comparison of MO orbitals
Vibration |
The comparison between the two techniques is very good. The orbitals are very similar. Therefore the computational method is very accurate. An upside to the computational method is you actual see the overlap of the orbitals from each fragment. Therefore being able to see the electrons spread easily.
cis and trans isomerisation
The optimisation of the cis and trans isomers of the Mo complex, [Mo(CO)4P(CH3)3], using the B3LYP method firstly using the LANL2MB basis set and reoptimised using the LANL2DZ.
Both the cis and trans isomers are not symmetrical as they both have C1 symmetry.
D space:
cis: http://hdl.handle.net/10042/to-1360 trans: http://hdl.handle.net/10042/to-1359
Energy of the cis isomer: -773.36024026 a.u. Energy of the trans isomer: -773.35731294 a.u. Energy difference: 0.0029273 a.u. = 6.632 KJ/mol
Bond length (Å): Mo-P Cis = 2.65193 Trans = 2.57186
Literature distances (Å): Mo-P Axial = 2.577 Equatorial = 2.576
The cis isomer bond length is longer than the literature as the two phosphine ligands are closer two as they are in the same plane therefore disfavoured interaction.
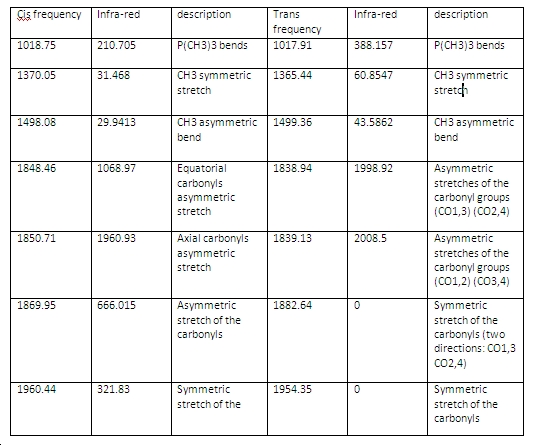
IR
D space:
cis: http://hdl.handle.net/10042/to-1362 trans: http://hdl.handle.net/10042/to-1361
Literature values of cis isomer: 2022, 1917, 1903 and 1882cm-1 Literature values of trans isomer: 1891 cm-1
As some of the frequencies the IR absorption for the trans isomer are not seen, as the carbonyl ligand stretches are in the same plane, therefore some of the asymmetric and symmetric stretches cancel out the dipole moments. Therefore not seen in the IR spectrum compared to the cis isomer which shows the IR stretches of these as a resultant dipole moment is formed as the carbonyls are axial as well as equatorial.
ammonia
symmetry
The main different in the structures is when looking at the d3h structure as the hydrogens are in the same plane s the nitrogen. The bond angle differs slightly from the other two ammonia structures. The calculation times only differed a little by a few seconds, the longest optimisation was the ammonia with the longer bond length. This is probably due to the fact the computer had to deal with a lower level symmetry.
If D3H it a sub group of C3v then going into more defined symmetry means calculation takes longer as its at a higher degree of accuracy. It suggests adopting a symmetry that isn’t the highest symmetry form indicates more rearrangements before the calculation can finish. Also if the molecule isn’t symmetrical the calculation takes longer as the case of the ammonia molecule with longer bond length.
When deciding whether or not a molecule can break it symmetry upon optimisation it depends on the adopting symmetry produced before the optimisation. Determining the symmetry before the optimisation can help and hinder it. A strained probably sub group point group will be probably push to break the symmetry if that symmetry doesn’t a mima energy when initial optimised. It all depends on if the the symmetry is already set on the molecule, because if another geometry (different) is more stable than the optimisation will change it.
methods
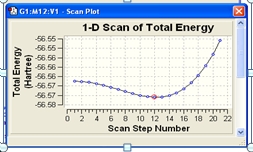
The MP2 optimisation calculation took only around 20 seconds longer than the the DFT method. But ammoina is a small moelcule therefore as the size increaes the time will increase in large amounts. ΔE=E(D3h)-E(C3v) in kJ/mol ΔE=(-56.426664911)-(-56.58263593)=0.15603102*2625.5= energy is too large, something wrong with my calculation, the value is ment to near 24.3KJ/mol
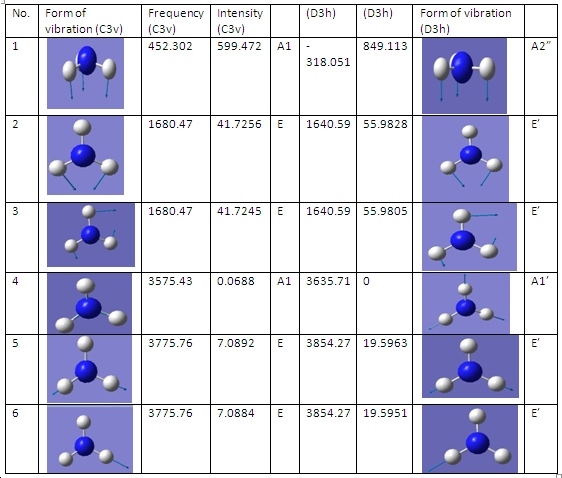
vibrations
+/- vibrations of the two symmetries C3v=all six vibratios are positive D3H=all are but one are positive
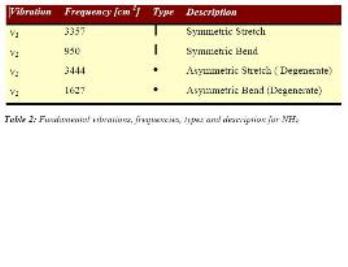
The C3v structure is the ground state structure; ground state structures always have all positive frequencies. All my values are positive same as the reference, the symmetric stretch and asymmetric bend frequencies are very close to the literature. The symmetric bend is very out (950 compared to 452) but this a small frequency but high intensity which suggests a different kind of bend vibration taking place. The negative frequency occurs in the D3h structure because this is how the programs conveys the convergence of two fragments, which is how the calculation optimisies a transition state. -318.051cm^-1 The D3h follows the inversion reaction path. As it is the transition the moves the lone pair electrons C3v to the empty p orbital, allowing the hydrogens to be in the same plane as the nitrogen. So the hydrogens can transfer from different planes to give mirror images C3v conformation.
mini project
In this project I will be looking at the Ni complexes, with the geometry of the ligands in a square planar geometry. Concentrating the coordination of the thiocyanate or isothiocyanate ligands, looking at the cis-trans isomer and stability of the two ligands. Preferably the other two ligands in the complex would be PPh3 ligands but these are two bulky to compute therefore to save time I have replaced the phenyl groups with methyl. Hence investigating the following structures: [Ni(PMe3)2(NCS)2] and [Ni(PMe3)2(SCN)2]. Also I will look at how changing the centre metal changes the stability of the complexes.
To the optimisations of the cis-trans isomers of the Ni complexes, for both the isothiocyanate and thiocyanate ligands using the B3LYP method and the basis set LANL2MB followed by the LANL2DZ when they were reoptimised.
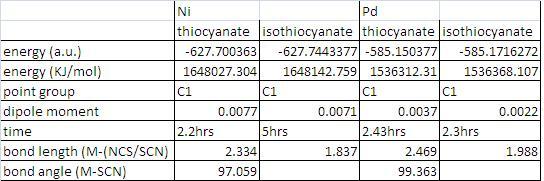
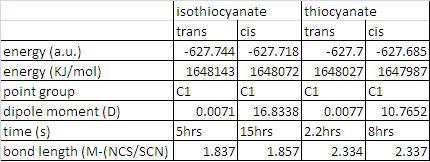 table showing inforamtion about the nickel complexes
table showing inforamtion about the nickel complexes
Cis Trans isomers
The major difference between the cis and trans isomers is the difference in dipole moments. The cis isomers have a large dipole moments, this must be because the geometry of the ligands are in square planar. Therefore when vibrations occur the opposite ligands will not cancel each other as they are different resulting in a dipole moment. The energetically stable seems to that in the trans conformation when looking as the dipole moment as it lower, also it is higher in energy. Bond lengths of the cis and trans isomers are obviously a longer in the cis conformation as it brings the more bulky PMe3 group closer together.
IR
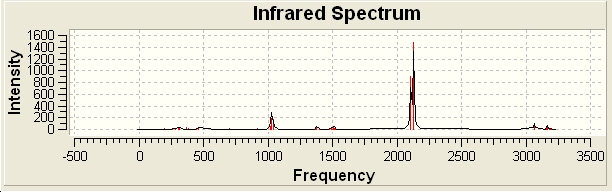
 IR spectra of the cis then trans isomers of the nickel complex with isothiocyanate coordination.
IR spectra of the cis then trans isomers of the nickel complex with isothiocyanate coordination.
If just looking at the infra-red frequencies of the isothiocyanate ligand of the cis and trans. The asymmetric stretches of the N=C vibrations are at the following frequencies: trans 2136.02 and cis 2107.63. They both have strong absorptions but if you at the symmetric stretches of the N=C vibrations, the trans isomer has an nonexistent absorption, 0.0027, as the ligands on the opposite of each other in the same plane. The frequencies are: trans 2147.12 and cis 2129.89.
NCS/SCN coordination
The geometry of the isothiocyanate and thiocyanate ligands as shown in the pictures above. See how the isothiocyanate ligands are in a perfect square complex with the PMe3 groups but the thiocyanate group are also square planar but the thiocyanate group is positioned axial.
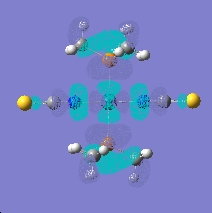
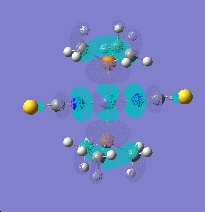
The bond lengths of coordination change a lot if it’s a N/S coordination. The bond is much shorter when the isothiocyanate ligand bonds. This is obviously due to the fact nitrogen is much smaller and I think only contributes a little to the stability of the ligands in the complexes, you need to at the molecular orbitals more clearly. When looking at the molecular there is a big difference between the HOMO and LUMO orbitals of the when looking at the different coordination of the ligands. The orbital overlap of thiocyanate group is much larger in the HOMO making the complex more stable than the isothiocyanate. The energy of the HOMO is -0.232 (SCN) and -0.199(NCS), which corresponds to the more stability of the (SCN) complex. This is probably due to the fact the sulphur is has more diffuse orbitals compared to nitrogen as it is period lower. Also the LUMO is lower in the thiocyanate complex.
LUMO- (thiocyanate)
(thiocyanate) (isothiocyanate)
(isothiocyanate)
HOMO- (thiocyanate)[[
(thiocyanate)[[ (isothiocyanate)
(isothiocyanate)
Difference in stability when changing the metal complex (by going down a period below Ni and producing a square planar complex with pallidium at the centre. )
From looking at the table above optimising the geometry of the thiocyanate, was slightly easily in the Ni complex, suggesting better coordination. If you look at the bond angles above it shows the thiocyanate ligand is at a lower angle in the nickel complex compared to Pd complex this would account for the slight increase optimisation time for the Pd complex. The opposite for the isothiocyanate ligand the time difference was much bigger, much shorter for the Pd complex.
When looking at the the HOMO orbitals of the two complexes there isn’t much difference the orbitals on Pd are slightly resulting in a slightly higher energy -2.04 (-0.209 for nickel complex) and the LUMOs are very close in energy this time Pd being very slightly lower at -0.089 ( and nickel at -0.088). Therefore you would expect the complex with Pd to have a lower energy, which is seen in the molecule above. Also to conclude that the Nickel complexes are energetically favourable than the Pd complexes, if you look at the bond lengths they are much shorter. Obviously because nickel is smaller metal, but shorter bonds means tighter bonds therefore more energetically stable.
references
1.http://www.ch.ic.ac.uk/hunt/teaching/teaching_MOs_year2/L3_Tut_MO_diagram_BH3.pdf
2.http://www.tesisenxarxa.net/TESIS_UB/AVAILABLE/TDX-1001103-104252//Appendix2.pdf -(NH3 IR frequencies)
3.Hogarth G, Norton T, Inorg. Chemica Acta, 254 (1997), 167-171.
4.A. D. Allen and P. F. Barrett, Can. J. Chem., 1968, 46, 1649.
5.DOI: 10.1021/ic00131a055