Rep:Mod:jhl416
Part 1
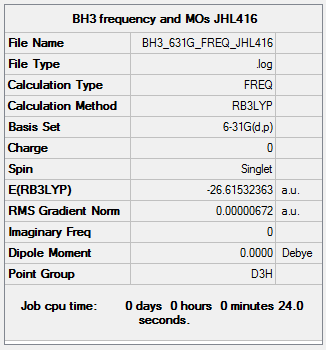
BH3
B3LYP/6-31G(d,p)
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000026 0.001200 YES
Frequency file: BH3_631G_FREQ_JHL416.log
Low frequencies --- -7.5477 -1.5512 -0.0054 0.6554 6.9821 7.1523 Low frequencies --- 1162.9679 1213.1635 1213.1662
Optimised molecule |
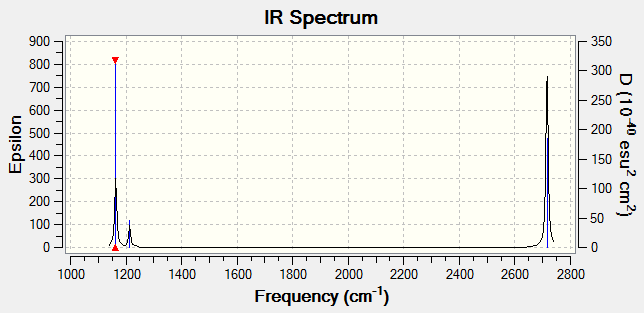
Vibrational spectrum for BH3
| Wavenumber (cm-1) | Intensity (arbitrary units) | IR active? | Type | |
| 1. | 1162 | 92 | Yes | Out-of-plane bend |
| 2. | 1213 | 14 | Slightly | Bend |
| 3. | 1213 | 14 | Slightly | Bend |
| 4. | 2582 | 0 | No | Symmetric stretch |
| 5. | 2715 | 126 | Yes | Asymmetric stretch |
| 6. | 2715 | 126 | Yes | Asymmetric stretch |
Ng611 (talk) 18:21, 30 May 2018 (BST) Good results! You should also include symmetry labels for each of the modes.
IR Spectrum:
Despite having 6 vibrations, there are only 3 vibrational peaks in the IR spectrum. This is due to firstly, the absence of vibration #4 at 2582(cm-1) as it is an IR inactive symmetric stretch. Secondly, there are 2 sets of degenerate vibrations, which are #2 & #3, and #5 & #6. The degenerate vibrations are observed as one single vibrational peak in the spectrum. Thus, only 3 peaks can be observed in the IR spectrum.
Ng611 (talk) 18:21, 30 May 2018 (BST) Good discussion!
MO diagram of BH3
The above shows the MO diagram of BH3 together with the 'real' MOs computed using GaussView. It can be seen that the LCAO MOs highly resembles the computed MOs. This indicates the high accuracy of the qualitative MO theory, which means it can be very useful to predict the shape of orbitals and molecules.
Ng611 (talk) 18:22, 30 May 2018 (BST) Very true. Are there any differences you can see at all?
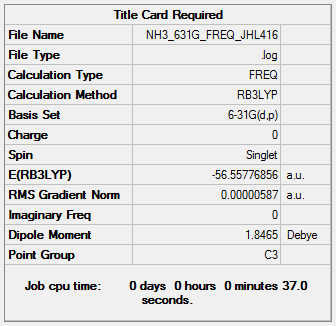
NH3
B3LYP/6-31G(d,p)
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Frequency file: NH3_631G_FREQ_JHL416.log
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Optimised molecule |
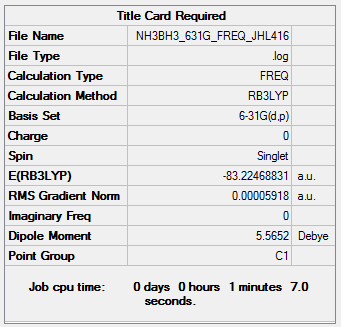
NH3BH3
B3LYP/6-31G(d,p)
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000114 0.000450 YES RMS Force 0.000059 0.000300 YES Maximum Displacement 0.000745 0.001800 YES RMS Displacement 0.000345 0.001200 YES
Frequency file: NH3BH3_631G_FREQ_JHL416.log
Low frequencies --- 0.0013 0.0014 0.0014 3.7980 18.8178 41.9105 Low frequencies --- 266.4229 632.1415 638.5829
Optimised molecule |
Energy determination
E(NH3)= -56.558 a.u.
E(BH3)= -26.615 a.u.
E(NH3BH3)= -83.225 a.u.
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -0.052 a.u. = -136.526 kJ/mol
Ng611 (talk) 18:23, 30 May 2018 (BST) You're off by a little bit here (I think it's probably a result of you going to 3 d.p instead of the recommended 5 d.p)
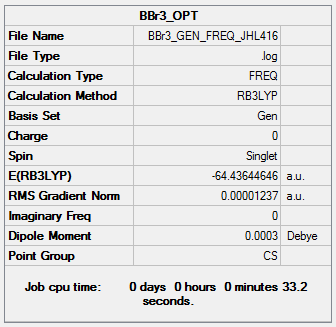
BBr3
B3LYP/6-31G(d,p)LANL2DZ
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000091 0.001800 YES RMS Displacement 0.000040 0.001200 YES
Frequency file: BBr3_GEN_FREQ_JHL416.log ( DOI:10042/202426 )
Low frequencies --- -5.9135 -3.4120 -2.3684 0.0002 0.0002 0.0002 Low frequencies --- 155.8395 155.9338 267.7016
Optimised molecule |
Part 2 Mini-project: Main group halides
Ng611 (talk) 18:25, 30 May 2018 (BST) You're missing your point groups here.
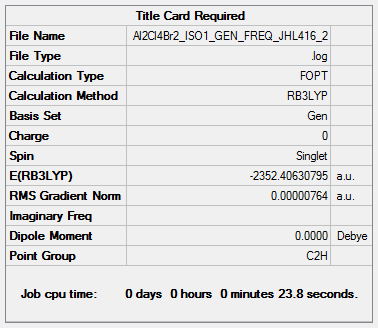
Isomer (1) (2 bridging Br ions)
B3LYP/6-31G(d,p)LANL2DZ
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000207 0.001800 YES RMS Displacement 0.000098 0.001200 YES
Frequency file: Al2Cl4Br2_ISO1_GEN_FREQ_JHL416_2.log ( DOI:10042/202436 )
Optimised molecule |
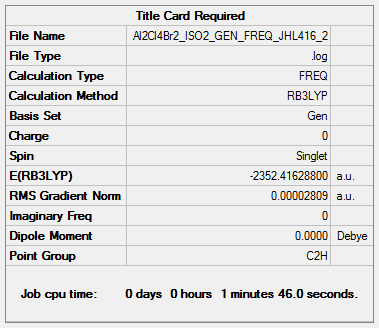
Isomer (2) (trans terminal Br and bridging Cl ions)
B3LYP/6-31G(d,p)LANL2DZ
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000069 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.001111 0.001800 YES RMS Displacement 0.000486 0.001200 YES
Frequency file: Al2Cl4Br2_ISO2_GEN_FREQ_JHL416_2.log ( DOI:10042/202438 )
Low frequencies --- -4.9115 -2.9474 -1.6704 -0.0019 -0.0012 -0.0008 Low frequencies --- 17.5237 48.9442 72.9542
Optimised molecule |
Relative stability
E(isomer 1)= -2352.406 a.u.
E(isomer 2)= -2352.416 a.u.
As isomer 2 lies at a deeper energy than the isomer 1, computed results show that isomer 2 is a more stable isomer. As Al and Cl both lies on the same period on the periodic table, there are better orbital overlap between the bond of Al and Cl than that between Al and Br. Thus, when Cl atoms are used as bridging ions, the overall energy of the molecule is more stable leads to a lower energy.
Ng611 (talk) 18:27, 30 May 2018 (BST) Good! I would actually calculate the energy difference between the molecules to strengthen this section further.
Determination of dissociation energy
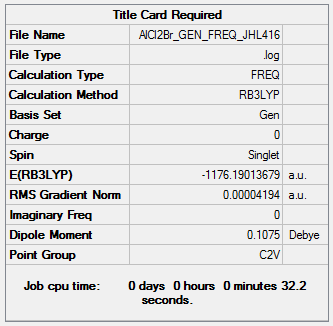
AlCl2Br
B3LYP/6-31G(d,p)LANL2DZ
Summary Table:
Item Value Threshold Converged? Maximum Force 0.000081 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001588 0.001800 YES RMS Displacement 0.000974 0.001200 YES
Frequency file: AlCl2Br_GEN_FREQ_JHL416.log ( DOI:10042/202440 )
Low frequencies --- -0.0055 -0.0052 -0.0050 1.3568 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Optimised molecule |
Calculations of dissociation energy from isomer 2 to 2AlCl2Br
E(AlCl2Br) = -1176.190 a.u.
E(Al2Cl4Br2) = -2352.416 a.u.
ΔE = 2 * E(AlCl2Br)- E(Al2Cl4Br2) = 0.036 a.u. = 94.518 kJ/mol
Ng611 (talk) 18:29, 30 May 2018 (BST) Remember to report your final value to the nearest Kj/mol.
The product is more stable as it is a positive change in energy.
Ng611 (talk) 18:29, 30 May 2018 (BST) What is the product here? Do you mean the dimer? Try to be more specific.
Analysis of 3 molecular orbitals from isomer 2
The following are 3 LACO MO diagrams using the computed results.
Ng611 (talk) 18:31, 30 May 2018 (BST) There's a slight contribution from the two terminal chlorine atoms that should be accounted for. It may be that they're a computational artefact, but you need to mention that you've treated them as such.
Ng611 (talk) 18:31, 30 May 2018 (BST) Good LCAO and Good decomposition
Ng611 (talk) 18:35, 30 May 2018 (BST) Good report. You let yourself down slightly by missing a couple parts out, and with a few accuracy errors when reporting your final results. Otherwise, a good effort. Well done.