Rep:Mod:jh46111
Part I
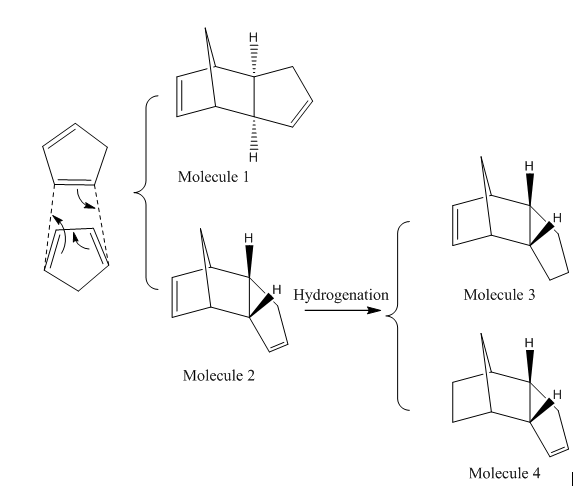
Hydrogenation of Cyclopentadiene Dimer
The Diels-Alder Reaction of two Cyclopentadiene molecules can form two forms of dimers, one is Endo (Molecule 1) and the other is Exo (Molecule 2). Molecule 3 and 4 are the products after hydrogenation. The fact that Endo form is the major product of the reaction is concluded from experiments. The energy of Endo, Exo forms and their hydrogenated forms can be calculated by Avogadro programme using energy calculation method MMFF94s; the energies are listed in Table 2.
| No. | Molecule 1(Endo) | Molecule 2(Exo) | Molecule 3 | Molecule 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
|
| Property | Molecule 1(Endo) | Molecule 2(Exo) | Molecule 3 | Molecule 4 |
|---|---|---|---|---|
| Total energy (kcal/mol) | 55.37342 | 58.19067 | 50.44566 | 41.34098 |
| Total electronic energy (kcal/mol) | 13.01368 | 14.18454 | 5.11949 | 5.14770 |
| Total VdW's energy (kcal/mol) | 12.80149 | 12.35877 | 13.63843 | 10.54000 |
| Total out-of-plane bending energy (kcal/mol) | 0.01475 | 0.02183 | 0.01311 | 0.00053 |
| Total torsional energy (kcal/mol) | -2.73093 | -2.94949 | -1.46888 | -0.31691 |
| Total stretch bending energy (kcal/mol) | -2.04135 | -2.08221 | -2.10211 | -1.64420 |
| Total angle bending energy (kcal/mol) | 30.77274 | 33.18926 | 31.93395 | 24.80178 |
| Total bond stretching energy (kcal/mol) | 3.54304 | 3.46797 | 3.31167 | 2.81207 |
After running the energy calculation of the 4 molecules listed above, 2 observations can be seen. 1. Total Energy of Molecule 1(Endo) is lower than the Total Energy of Molecule 2(Exo). Comparing different kinds of energies, it has been found that the main difference is due to the angle bending energy, 30.77274kcal/mol vs 33.18926kcal/mol. 2. Total Energy of Molecule 3 is higher than that of Molecule 4. The main difference is also due to angle bending energy, 31.93395kcal/mol vs 24.80178kcal/mol.
The natural occurring bond angles are sp2 and 109.5° in sp3; the angles in the molecules differ from the natural occurring ones are due to the angle bending energy. By measuring the bond angles in the molecules, the deviations can be concluded in the Table 3. It is shown clearly that there is more Angle Deviation of Molecule 1 than that of Molecule 2; more Angle Deviation of Molecule 3 than Molecule 4.
| Property | Angle 1 | Angle 2 | Angle 3 |
|---|---|---|---|
| Atoms involved | C3,C6,C8 | H19,C8,C6 | C5,C4,C7 |
| Natural occurring | 109.5 | 109.5 | 109.5 |
| Angle/Deviation (Molecule 1) | 114.9 (+5.4) | 110.5 (+1) | 102.5 (-7) |
| Angle/Deviation (Molecule 2) | 117.8 (+8.3) | 113.0 (+3.5) | 100.2 (-9.3) |
| Property | Angle 1 | Angle 2 | Angle 3 |
|---|---|---|---|
| Atoms involved | C3,C6,C8 | H19/22,C8,C6 | C5,C4,C7 |
| Natural occurring | 109.5 | 109.5 | 109.5 |
| Angle/Deviation (Molecule 3) | 118.8 (+9.3) | 111.7 (+2.2) | 100.1 (-9.4) |
| Angle/Deviation (Molecule 4) | 118.6 (+9.1) | 110.5 (+1) | 101.1 (-8.4) |
The reason that Endo form is the major product in experiment is due to the lower energy of Transition state of Endo form. Endo form has higher energy which mean it is more unstable. This Diels-Alder reaction is under kinetic control instead of thermodynamic control as the more unstable Endo form is the major product.
Molecule 3 & 4 are the production of hydrogenation from Molecule 2. The Total Energies of Molecule 3 & 4 are both lower than that of Molecule 2. It means the hydrogenation is favored.
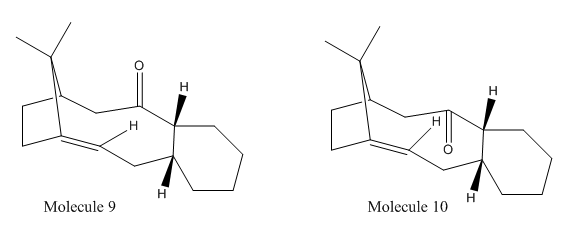
Molecule 9 and 10 [1]are atropisomers and they give Molecule 9(saturated) and 10(saturated) after hydrogenation. Comparison can be done between the saturated form and unsaturated form of 9 and 10.
| No. | Molecule 9 | Molecule 10 | Molecule 9 (saturated) | Molecule 10 (saturated) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
|
| Property | Molecule 9 | Molecule 10 | Molecule 9 (saturated) | Molecule 10 (saturated) |
|---|---|---|---|---|
| Total energy (kcal/mol) | 70.54467 | 66.29108 | 81.75656 | 73.77833 |
| Total electronic energy (kcal/mol) | 0.30395 | -0.06889 | 0.0000 | 0.0000 |
| Total VdW's energy (kcal/mol) | 33.11930 | 35.06832 | 32.71947 | 32.80239 |
| Total out-of-plane bending energy (kcal/mol) | 0.97422 | 0.25399 | 70.54467 | 0.23332 |
| Total torsional energy (kcal/mol) | 0.31308 | 3.65335 | 9.47264 | 9.21881 |
| Total stretch bending energy (kcal/mol) | -0.08187 | 0.30222 | 0.30222 | 0.32484 |
| Total angle bending energy (kcal/mol) | 28.25720 | 32.05685 | 32.05685 | 24.29611 |
| Total bond stretching energy (kcal/mol) | 7.65878 | 6.95138 | 6.95138 | 6.90286 |
| Property | Angle 1 | Angle 2 | Angle 3 |
|---|---|---|---|
| Atoms involved | C7,C3,C6 | H18,C11,C5 | C5,C4,C7 |
| Natural occurring | 109.5 | 109.5 | 120 |
| Angle/Deviation (Molecule 3) | 118.5 (+9) | 107.9 (+1.6) | 123.4 (-3.4) |
| Angle/Deviation (Molecule 4) | 116.4 (+6.9) | 108.8 (+0.7) | 122.3 (-2.3) |
Same arguments here as of Molecule 1 to 4. Molecule 9 has higher energy than Molecule 10, among all types of energies, total angle bending energy contributes the most. It means the angles deviated more for Molecule 9, the details of deviations show in the table 7.
Molecule 9 & 10(saturated) are the products of hydrogenation of Molecule 9 & 10 respectively. Olefin strain[2] energy should be taken into account in this case. Olefin strain is the difference between the strain energy of olefin and its saturated product. The strain energies of saturated molecules are higher than the unsaturated molcules. This is because olefins are locating in the brigehead of the molecules and as a result, the rate of hydrogenation from Molecule 9 & 10 to their saturated forms is low.
Derivative Molecules from Taxol
| No. | Molecule 17 boat | Molecule 17 chair | Molecule 18 boat | Molecule 18 chair | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
|
| Property | Molecule 17 boat | Molecule 17 chair | Molecule 18 boat | Molecule 18 chair |
|---|---|---|---|---|
| Total energy (kcal/mol) | 114.33657 | 104.98391 | 102.28716 | 100.46235 |
| Total electronic energy (kcal/mol) | -7.05008 | -7.22117 | -6.24215 | -6.05131 |
| Total VdW's energy (kcal/mol) | 54.37213 | 52.21497 | 50.49934 | 49.45308 |
| Total out-of-plane bending energy (kcal/mol) | 1.18784 | 1.02419 | 96.99088 | 0.90018 |
| Total torsional energy (kcal/mol) | 14.28618 | 10.98776 | 13.58885 | 9.76322 |
| Total stretch bending energy (kcal/mol) | 0.76536 | 0.31639 | 0.48412 | 0.63651 |
| Total angle bending energy (kcal/mol) | 35.01470 | 31.52244 | 28.54054 | 30.70962 |
| Total bond stretching energy (kcal/mol) | 15.89062 | 15.97568 | 14.39227 | 15.05105 |
It can be concluded from Table 8 that Molecule 17 has higher energy than Molecule 18 and the chair forms have lower energy than the boat forms.
Table 10 shows the energies of Molecule 17 & 18 chair forms.
| Property | Molecule 17 chair | Molecule 18 chair |
|---|---|---|
| Sum of electronic and thermal Energies (kJ/mol) | -1615.412 | -1651.417 |
| Sum of electronic and thermal Energies (kJ/mol) | -1615.390 | -1651.396 |
| Sum of electronic and thermal Enthalpies (kJ/mol) | -1615.389 | -1651.395 |
| Sum of electronic and thermal Energies (kJ/mol) | -1615.459 | -1651.464 |
| No. | Molecule 17 NMR | Molecule 18 NMR |
|---|---|---|
| Structure | 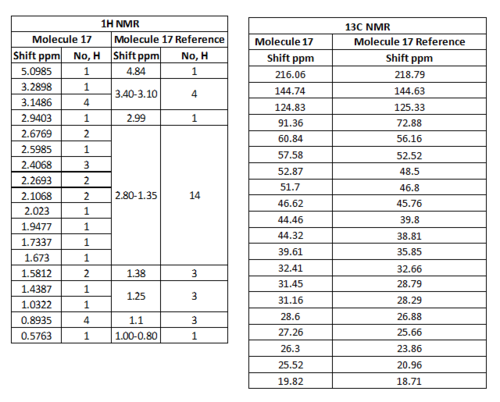 |
|
| No. | 1H NMR | 13C NMR |
|---|---|---|
| Spectrums | 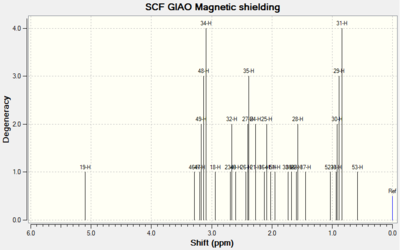 |
 |
| No. | 1H NMR | 13C NMR |
|---|---|---|
| Spectrums | 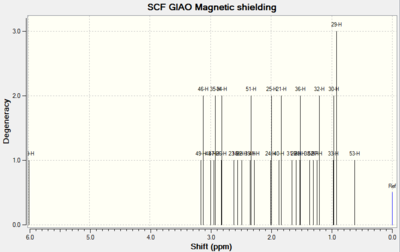 |
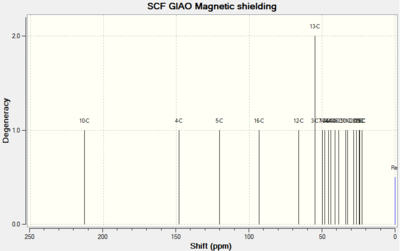 |
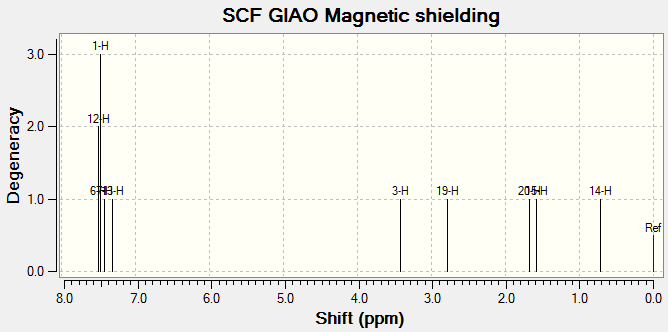
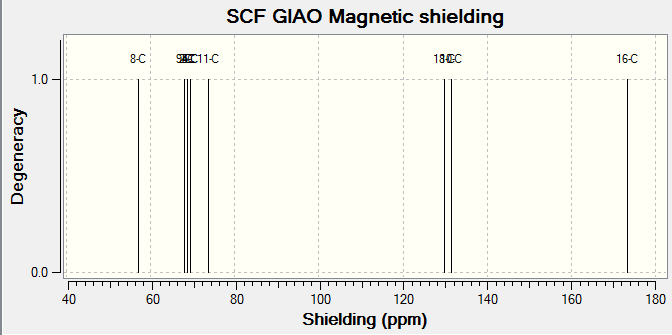
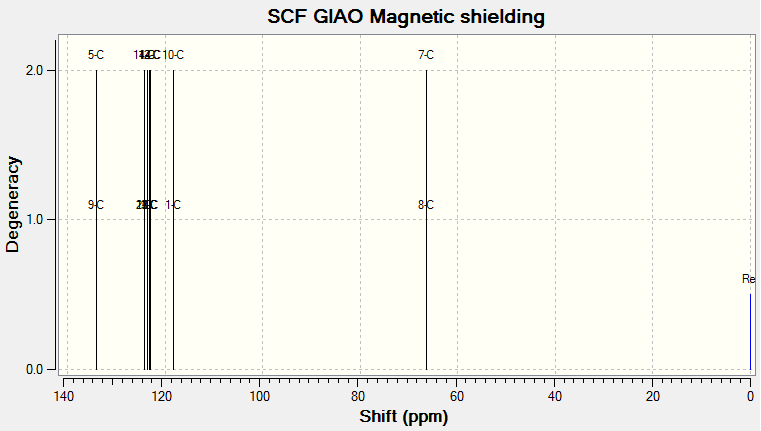
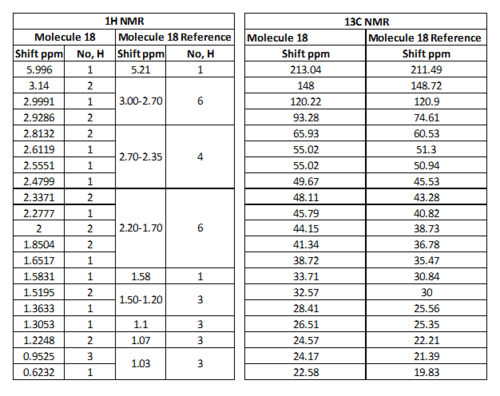
For Table 11, the NMR data are calculated by first optimising energy by Avogadro then introduced to Gaussian. B3LYP/6-31G (d,p)basis, chloroform solvent are used.
By comparing 1H NMR spectra, the computed chemical shifts are generally larger than the chemical shifts in literature[3]. The hydrogen atoms on the methyl group near carbonyl bond are more desheided. For 13C NMR, the computed chemical shifts match the those in literature.
Part 2
Two Catalysts
Properties analysis of alkene epoxide
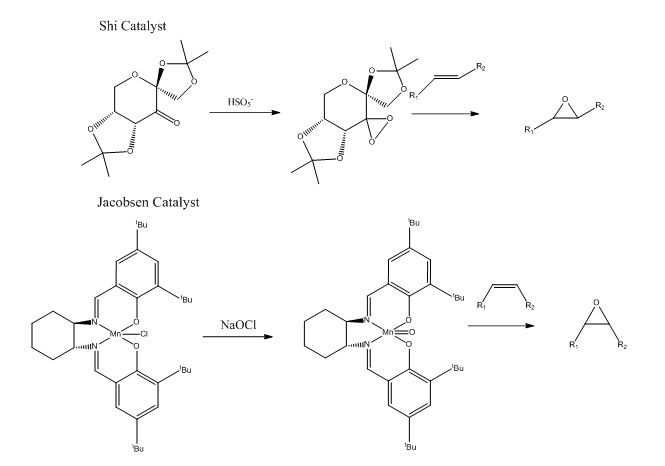
Two catalysts can be used to epoxide alkene: Shi [4]and Jacobsen catalyst[5].
For Shi catalyst, the C-O bond length in the anomeric centre is different.
| Structure | 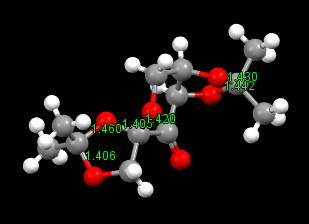 |
|
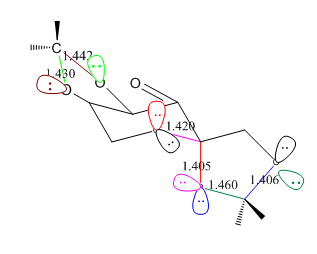
|
The C-O bond length in the Shi catalyst varies and differs from the ideal C-O single bond length: 1.43Å. There are three O-C-O centres and the bond lengths are shown in the graph above.
The lone pair on oxygen can overlap with the anti-bonding orbital of C-O bond when the orientation is anti-periplanar: LPO/σ*C-O. There are six pairs of overlap in total which are shown on the graph above.
The C-O bond will compress when its its oxygen donates lone pair; the C-O bond will extend when other oxygen donates lone pair. This can answer the question why the C-O bond is different within the same anomeric centre.
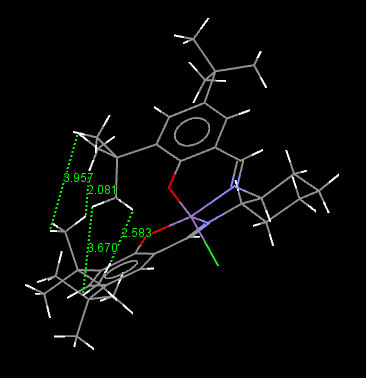
For Jacobsen catalyst, the structure can be depicted as square pyramid.
ah |
For a 5-coordinated Mn, trigonal bipyramidal should be the ideal stucture. Here for Jacobsen catalyst, square plane pyramid is preferred due to the steric effect. The H atoms in the tBu group close to each other, the distances are shown in the graph which are slightly larger than the two times of the van der Waals radius of hydrogen: 2 x 1.20Å =2.40Å. These are attraction interaction which can stabilize the molecule.
Two alkenes
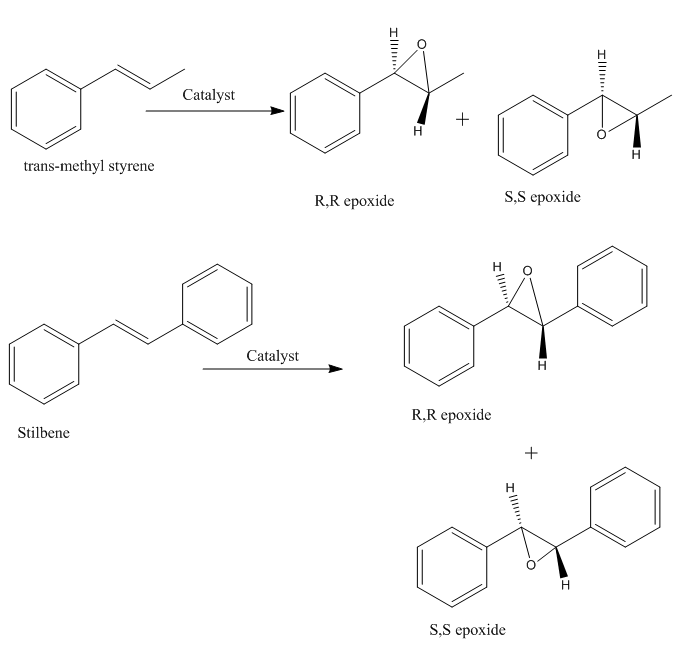
The following 2 alkenes can be oxidized to epoxide when treated with catalyst.
The epoxides of methyl styrene and stilbene have two forms: R,R and S,S. Here are the structures in 3D.
| No. | methyl styrene oxide R,R | methyl styrene oxide S,S | Stilbene oxide R,R | Stilbene oxide S,S | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
|
The 1H and 13C NMR of tran-methyl styrene oxide and Stilbene oxide are listed below.
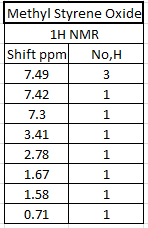
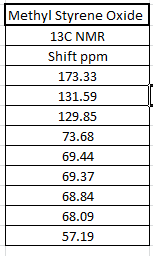
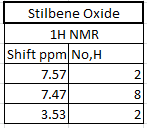
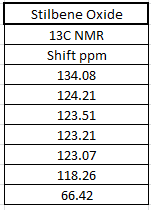
The alkene that under epoxiation can remain the E/Z stereochemistry. However, the epoxiation has enantimochemistry and the result product of the epoxide can be assigned as R,R or S,S (epoxidation is syn-reaction). As a result, techniques can be used to determine the stereochemistry of the final product. There are three ways can be used to show which enantiomer resulted: Reaxys used to know the optical rotation value in literature, calculated chiroptical properties of the product and properties of transition state for the reaction.
The reported literature for optical rotations
Literature from Reaxys, the optical rotation:
| methyl-Styrene oxide | R,R | S,S |
|---|---|---|
| Optical rotation | 44.3 deg [6] | -41.8 deg [7] |
| Concentration | 0.32 g/100ml | 1g/100mL |
| Temperature | 25°C | 25°C |
| Solvent | chloroform | chloroform |
| Wavelength | 589nm | 589nm |
| Siltbene oxide | R,R | S,S |
|---|---|---|
| Optical rotation | 296 deg [8] | -291 deg [9] |
| Concentration | 1.01 g/100ml | 0.6g/100mL |
| Temperature | 22°C | 15°C |
| Solvent | ethanol | acetone |
| Wavelength | 589nm | 589nm |
The calculated chiroptical properties of the product
By comparing the values of the computed chiroptical properties and the values gained from the experiment or literature, the specified enantiomer of epoxides can be predicted. The three chiroptical properties are: 1. The optical rotation at specified range of wavelength. 2. The electronic circular dichroism (ECD) 3. The vibrational circular dichroism(VCD)[10]
1.The QM-Optimized geometry is used and input into Cambridge variation on B3LYP density functional method. Polar(optrot) calculated the [alpha]D optical rotation components of the epoxides. [alpha]D optical rotation at wavelength range 589nm has the following vaules:
Methyl-styrene oxide, R,R & S,S structures have optical rotation: [Alpha] ( 5890.0 A) = 46.78 deg and [Alpha] ( 5890.0 A) = -46.78 deg repectively. Silbene oxide, R,R & S,S structures have optical rotation: [Alpha] ( 5890.0 A) = 297.68 deg[Alpha], and(5890.0 A) = -298.24 deg respectively. Both of the optical rotation data roughly match the literature data.
| methyl-Styrene | R,R (computed) | S,S (computed) | R,R (literature) | S,S (literature) |
|---|---|---|---|---|
| Optical rotation | 46.78 deg | -46.78 deg | 44.3 deg | -41.8 deg |
| Siltbene oxide | R,R (computed) | S,S (computed) | R,R (literature) | S,S (literature) |
|---|---|---|---|---|
| Optical rotation | 297.68 deg | -298.24 deg | 296 deg | -291 deg |
2.The ECD is useless for epoxides as there are no chromophore these epoxides
3.The VCD spectrum of methyl-styrene and siltbene and their oxides are attached here:
| No. | methyl styrene oxide R,R | methyl styrene oxide S,S |
|---|---|---|
| Structure |  |
 |
| No. | Stilbene oxide R,R | Stilbene oxide S,S |
|---|---|---|
| Structure |  |
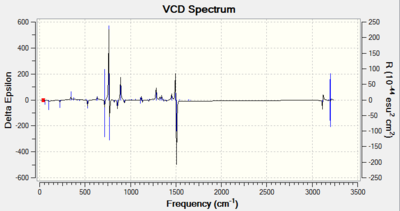 |
As it can be seen from the VCD Spectrum above, the pair of enantimoers have similar but opposite spectrum. Since the R,R and S,S conformers are mirror images to each other, the vibrations in VCD Spectrum is opposite as well. This technique can be used to assign the stereochemistry of the molecule but it is not available in Imperial College.
Using the (calculated) properties of transition state for the reaction
The enantiomeric excess and relative population of the two two conformers of a molecule can be calculated by the following method.
The following is the transition state energies of the reaction Jacobsen catalyst and trans-methyl styrene.
| enantimoers | R,R [11] | S,S[12] |
|---|---|---|
| Sum of electronic and zero-point Energies (Hartree) | -3383.183068 | -3383.188478 |
| Sum of electronic and thermal Energies (Hartree) | -3383.141188 | -3383.145943 |
| Sum of electronic and thermal Enthalpies (Hartree) | -3383.140244 | -3383.144999 |
| Sum of electronic and thermal Free Energies (Hartree) | -3383.254344 | -3383.262481 |
| Free energy Difference (Hartee) | -0.008137 | |
| Free energy Difference (kJ/mol) | -21.36367 | |
| Equilibrium Constant K | 5533.018 | |
| Enantiomeric excess | 99.98% |
ΔG=-RTlnK is the equation applied here. R is ideal gas constant= 8.314J/mol*K[13]. T is the temperature, room temperature 298.15K is used here. Equilibrium constant K can be calculated: 5533.018. The S,S conformation is a in large excess. The enantiomeric excess of S,S conformation is 99.98%.
The following is the transition state energies of the reaction Jacobsen catalyst and cis-methyl styrene.
| enantimoers | S,R | R,S |
|---|---|---|
| Sum of electronic and zero-point Energies (Hartree) | -3383.185100 | -3383.177857 |
| Sum of electronic and thermal Energies (Hartree) | -3383.142495 | -3383.135845 |
| Sum of electronic and thermal Enthalpies (Hartree) | -3383.141551 | -3383.134901 |
| Sum of electronic and thermal Free Energies (Hartree) | -3383.259559 | -3383.250270 |
| Free energy Difference (Hartee) | -0.009289 | |
| Free energy Difference (kJ/mol) | -24.388 | |
| Equilibrium Constant K | 18744.52 | |
| Enantiomeric excess | 99.99% |
ΔG=-RTlnK is the equation applied here. Equilibrium constant K can be calculated: 18744.52. The S,R conformation is a in large excess. The enantiomeric excess of S,R conformation is 99.99%.
| R,R | S,S | |
|---|---|---|
| Literature | 90 ee | 99 ee |
| Through Calculation | 99.98 ee [14] | 99.98 ee [15] |
The data in the literature is smaller than that obtained from the experiments. The data obtained through calculation doesn't take solvents and other conditions into consideration.
Non covalent interaction analysis
Orbital |
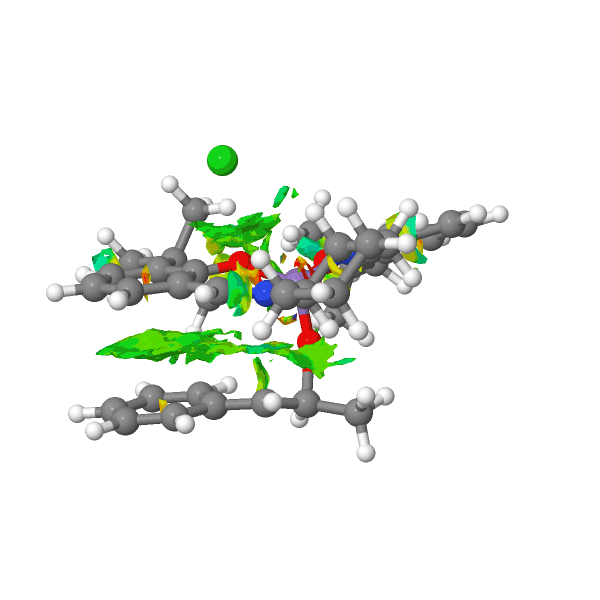
This image is the Non covalent interaction electron cloud analysis of the transition state of tran-methyl styrene reacting with Jacobsen catalyst. Colour green in the graph means interaction and red means repulsion. As it is shown in the graph, the major colour is green. The alkene is reacting with the catalyst by maximizing the interaction. The red atom in the catalyst is bonding to the double bond of methyl styrene. From the orientation of the two reactants in the transition state, the product can be predicted to be S,S conformation.
The following is the reaction scheme that can predicted from the transition state graph above.
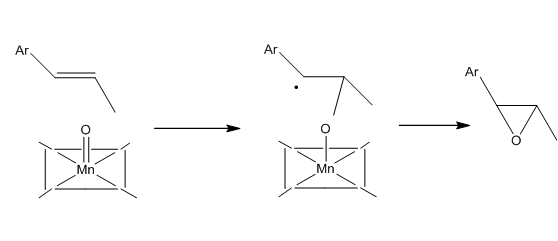
QTAIM Analysis in the active-site of the S,S conformer from Jacobsen catalyst and tran-methyl sytrene reaction transition state
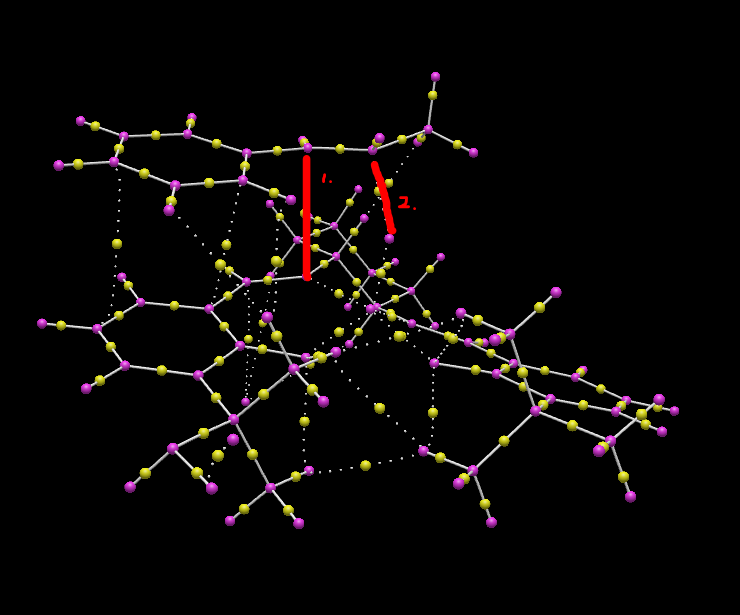
BCP is known as bond critical point, which means the first derivatives of the electron cloud is zero at that point. The first red line noted in the graph is the bond forming interaction of C and O. The second red line noted in the graph is the interaction of N and C. These two interaction can determine the stereochemistry of the product.
Suggesting new candidates for investigations
| enantimoers | Conformer 1 S [16] | Conformer 2 R [17] |
|---|---|---|
| Structure | 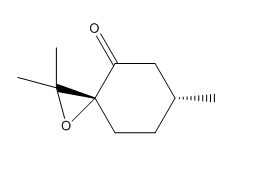 |
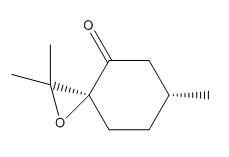
|
| Name | pulegone epoxide | cis R-(+)-pulegone oxide |
| Solvent | ethanol | ethanol |
| Wavelength (nm) | 324 | 324 |
| Optical Rotation Power | -1177.9 deg | 853.9 deg |
| Concentration (g/100mL) | 0.03 | 0.03 |
| Temperature(°C) | 25 | 25 |
By searching the Sigma-Aldrich page, both (S)-(-)-pulegone and (R)-(+)-Pulegone can be found. The two epoxides above are both able to be synthesized.
Reference
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319;DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891DOI:10.1021/ja00398a003
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ O. A. Wong , B. Wang , M-X Zhao and Y. Shi J. Org. Chem., 2009, 74, 335–6338;DOI:10.1021/jo900739q
- ↑ M. Palucki , N. S. Finney , P. J. Pospisil , M. L. Güler , T. Ishida , and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 948–954; DOI:10.1021/ja973468j
- ↑ Wong,O.Andrea;Wang,Bin ;Zhao,Mei-Xin and Shi,Yian,Journal of Organic Chemistry, 2009, 74, 6335-6338. DOI:10.1021/jo900739q
- ↑ Lin,Hui;Liu,Yan;Wu,Zhong-Liu,Tetrahedron:Asymmetry, 2011, 22, 134-137.DOI:10.1016/j.tetasy.2010.12.022
- ↑ Solladie-Cavallo, Arlette; Diep-Vohuule, Anh; Sunjic, Vitomir; Vinkovic, Vladimir Tetrahedron: Asymmetry, 1996 , vol. 7, # 6 p. 1783 - 1788DOI:10.1016/0957-4166(96)00213-3
- ↑ Read; Campbell Journal of the Chemical Society, 1930 , p. 2377 Nature (London, United Kingdom), 1930 , vol. 125, p. 16DOI:10.1039/jr9300002377
- ↑ M. J. Fuchter, Ya-Pei Lo and H. S. Rzepa, J. Org. Chem., 2013,
- ↑ DOI:10.6084/m9.figshare.856651
- ↑ DOI:10042/25945
- ↑ http://physics.nist.gov/cgi-bin/cuu/Value?r
- ↑ Wong,O.Andrea;Wang,Bin ;Zhao,Mei-Xin and Shi,Yian,Journal of Organic Chemistry, 2009, 74, 6335-6338. DOI:10.1021/jo900739q
- ↑ Lin,Hui;Liu,Yan;Wu,Zhong-Liu,Tetrahedron:Asymmetry, 2011, 22, 134-137.DOI:10.1016/j.tetasy.2010.12.022
- ↑ Reusch; Johnson Journal of Organic Chemistry, 1963 , vol. 28, p.2557{{DOI|10.1021/jo01045a016}
- ↑ Reusch; Johnson Journal of Organic Chemistry, 1963 , vol. 28, p.2557{{DOI|10.1021/jo01045a016}