Rep:Mod:jgh001
Julia Gonzalez Holguera 3rd Year Computational Labs
Molecular modelling is a computer based technique which allows, upon theoretical grounds, to optimise structures of molecules and determinine some of their properties. Different computational methods have been developed and are now widely used by chemists to rationalize and predict structure and reactivity of molecules. Molecular mechanics (MM) methods allow to study molecular properties, not from a quantum mechanical treatment but by summation of individual bond properties. Quantum mechanical methods, in the other hand, provide explicit solutions for the electronic behaviours of a molecule. This module aims to introduce some of the techniques available to perform computational modelling. Molecular mechanics will be use first, to predict the geometry and regioselectivity of various molecules and illustrate some of the method’s limitations. Semi-empirical and density functional molecular orbital methods will then be used to investigate the regioselectivity of the electrophilic carbenylation of a chloro-substituted bicyclic diene, and to investigate the effect of neighboring group participation. A mini-project will finally be undertaken, to investigate how spectroscopic simulations of organic molecules can be obtained from computational methods, based upon an example chosen from the primary literature.
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
This section will make use of the Allinger MM2 molecular mechanics model implemented in the ChemBio3D program.
Discussion
Cyclopentadiene is an organic molecule which is known to dimerise at room temperature via a reversible Diels-Alder reaction and to dissociate back to cyclopentadiene at temperatures above 160 ºC [1]. Interestingly, the dimerization reaction produces specifically the endo dimer rather than the exo dimer. This module will aim to understand the reasons behind this stereoselectivity. The structures of the two dimers were drawn and optimized in ChemBio 3D using the MM2 method. The contributions to the total energies of the two molecules are reported in Table 1. The method, which predicts that the exo product is thermodynamically preferred, cannot rationalize the preference observed for the endo product.
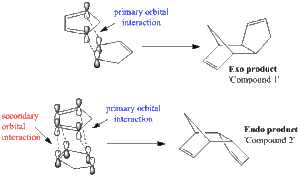
The geometrical alignment, in the transition state, of the two fragments has to be considered to explain the stereoselectivity observed in the Diels Alder cycloaddition. Figure 2 illustrates the two ways in which the cyclopentadiene monomers can align themselves before dimerisation, and in doing so generating either the endo or the exo dimer. Both mechanisms involve primary orbital overlap between the suitable HOMO and LUMO orbitals. However, the endo dimerization features an additional secondary orbitals overlap, which stabilises the transition state of the endo dimer relative to the exo one, and therefore makes the endo product kinetically favoured. Therefore, under non-equilibrating conditions, one would expect the endo product to be favoured, despite being thermodynamically less stable than its exo conformer.
| Conformer | Energy (kcal/mol) |
|---|---|
| Exo product | 31.8766 |
| Endo product | 33.9975 |
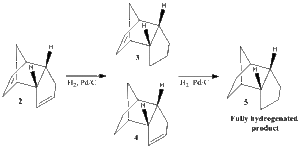
Hydrogenation of the preferred cyclopentadiene dimer can be obtained by exposure to hydrogen in the presence of Pd/C catalyst. This proceeds to give initially a partially hydrogenated product, after which the second double bond reacts and the tetrahydro derivative is formed. Using an MM2 method, the energies of the two possible monohydrogenated intermediates 3 & 4 were calculated and are presented in Table 2. The data obtained shows that intermediate 4 is thermodynamically preferred. Assuming the hydrogenation is under thermodynamic control, intermediate 4 is expected to be formed, before further reacting and forming the fully hydrogenated product 5.
| Molecules | Total energies (kcal/mol) | Stretching | Bending | Torsion | 1,4 VdW | Dipole/Dipole |
|---|---|---|---|---|---|---|
| 3 | 35.6898 | 1.2794 | 19.7572 | 10.8773 | 5.6288 | 0.1621 |
| 4 | 31.1621 | 1.1063 | 14.5110 | 12.5103 | 4.4995 | 0.1407 |
| Intermediate 3 | intermediate 4 |
|---|---|
|
|
3 & 4 differ from the location of their double bond and any difference in energy between the two structures should therefore originate from this double bond. In isolated monoalkene rings, the molecules are less strained when the double bond is part of a 6 rather than a 5 membered ring. However 4 is found to be more stable than 3. This can probably be explained by the presence of the alkyl bridge, which constrains the angles of the 6-membered ring. The C=C-C bond angles for 3 and 4 are 108• and 113• respectively, when the ideal angle for sp² carbons is 120•.
Conclusion
The MM2 method was used to study the dimerisation and hydrogenation of cylopentadiene. Some of the limitations of using a molecular mechanic method on its own were uncovered. The energies obtained could not rationalise the stereospecificity observed experimentally for the dimerisation reaction. An explanation based upon Frontier Molecular Orbital was provided, suggesting that the endo product, though thermodynamically not preferred, was none-the-less the kinetically preferred product. The hydrogenation reaction was assumed to be under thermodynamic control, and since 4 was found to be more stable than 3, 4 was determined to be the intermediate in the hdyrogenation process.
References
1. Dieter Hönicke, Ringo Födisch, Peter Claus, Michael Olson, Cyclopentadiene and Cyclopentene, Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. DOI:10.1002/14356007.a08_227
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
Discussion

Taxol is a molecule which is used as an anti-cancer agent. Compounds 9 and 10 are two atropisomeric intermediates in its synthesis, which differ by the direction in which the carbonyl group is pointing. The two compounds are in equilibrium, and the stereochemistry of the carbonyl therefore depends on the thermodynamically preferred conformation. Molecular mechanics MM2 force field method was used to determine the energy of the two isomers and therefore determine which of the two is preferred. The data obtained is reported in Table 3.
| Compound 9 (boat) | Compound 9 (chair) | Compound 10 (boat) | Compound 10 (chair) | |
|---|---|---|---|---|
| Total Energy (kcal/mol) | 54.60 | 50.50 | 50.51 | 48.30 |
| Compound 9 | Compound 10 | |
|---|---|---|
| Representations | Taxol intermediate carbonyl up | Taxol intermediate carbonyl down |
| Total Energy (kcal/mol) | 74.67 | 70.73 |
The first results obtained using the MM2 method showed the cyclohexane in its boat conformation. As it is known that the chair conformation is usually preferred over the boat one for cyclohexane, the structures were modified by manual editing to force the program to come up with the chair conformations of the two isomers. As expected, this further reduced the energies of the two isomers. The data obtained, as well as a representaion of the two isomeric structures are reported in Table 3. The energies obtained from the MM2 method were compared with those obtained using the MMFF94 method. The absolute values differ from one method to the other. However, the relative energies between the two isomers were similar with either method.
The results obtained from both methods suggest that the structure of compound 10, featuring its carbonyl group pointing down, is lower in energy than that of compound 9. Compound 10 is therefore the thermodynamic product and one can expect the subsequent steps in Taxol synthesis to have the carbonyl functional group pointing down. A visual inspection of the structure of compound 9 shows that the carbonyl is indeed likely to come into steric clash with the bridging methyl substituents.
It was observed that during subsequent functionalization of the alkene, the double bond reacted abnormally slowly. This is an example of an hyperstable alkene. Hyperstable alkenes were first described by Schleyer in 1981 [1] and are characterized by their reluctance to undergo further chemical reactions. The olefin strain energy (OS) was defined as the difference between the strain energy of an olefin and that of its parent hydrocarbon. It is generally found that the strain energy of a cycloalkene is higher than that of its parent cycloalkane. However, some bridgehead olefins happen to be less strained than their parent hydrocarbon and these are referred to as hyperstable. A set of empirical rules were proposed by Schleyer & co to distinguish three groups of bridgehead olefins.
- isolable bridgehead olefins: OS < 17 kcal/mol
- observable bridgehead olefins: 17 < OS < 25 kcal/mol
- unstable bridgehead olefins: OS> 25 kcal/mol
Here the parent hydrocabon of compound 10 is found to have an energy of 51.36 kcal/mol (obtained from MM2 method). Therefore, this suggests an OS of -3.1 kcal/mol. This means that the present compound 10 falls in the category of the isolable bridgehead olefins, which is indeed what is found. This explains the observed low reactivity of the double bond.
Conclusion
The stability of two isomers were calculated using two different molecular mechanics methods, which both suggested that 10 was more stable than 9. The hyperstability of the molecule was discussed in relation to its Olefin Strain (OS).
References
1. P.v.R. Schleyer, J.Am.Chem.Soc., 1981, 103, 1891-1900, DOI:10.1021/ja00398a003
2. A. McEwen, P.v.R. Schleyer, J. Am. Chem. Soc., 1986, 108 (14), pp 3951–3960, DOI:10.1021/ja00274a016
3. P.Camps, F.Perez, S.Vasquez, Tetrahedron, Vol. 53, No. 28, pp. 9727-9734. 1997, DOI:1016/S0040-4020(97)00595-4,
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
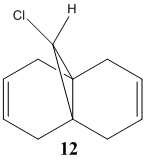
Discussion
This section aims to illustrate why an explicit consideration of the electrons is necessary when predicting the reactivity of molecules, and also how electrons influence bonds and the derived spectroscopic properties. It is reported in the litterature that compound 12 undergoes reaction with electrophiles regioselectively on the π-face endo to the chlorine. Part 1 will aim to rationalise this observation, using a quantum mechanical treatment which includes the wave-descrition of the electrons. Part 2 will aim to investigate the influence of the C-Cl bond strength on the vibrational frequencies of the molecule. Finally, Part 3 will illustrate the effects that different susbtituents placed on the exo double bond can have on the vibrational spectra of the molecule.
Part 1
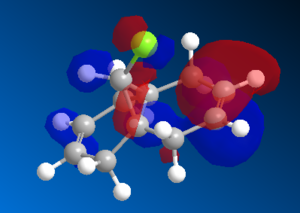
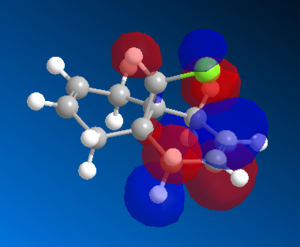

The geometry was optimised using the MM2 method. The MOPAC/PM6 method was then used to generate an approximation of the valence electron molecular wavefunctions. The orbitals generated, however, were found to be a poor reprensentation of the molecule as they did not respect the symmetry of the molecule. These were therefore treated as incorrect and due to some problem in the method used. The calculations were repeated using the MOPAC/RM1 and the MOPAC/PM3 methods instead. The orbitals generated in both cases respected the geometry of the molecule, so the PM6 method was assumed to be the origin of the incorrect orbitals previously obtained.
The HOMO orbitals are the one supposed to be the most reactive towards electrophilic attack. Their representations obtained from the MOPAC/PM6, MOPAC/RM1 and the MOPAC/PM3 methods are reported on the left. The orbitals generated from the last two methods are symmetrical with respect to a symmetry axis which passes through the middle of both double bonds.
The diene's regioselectivity towards electrophilic addition have been discussed in the litterature and attributed to a stabilizing antiperiplanar interaction between the σ*(Cl-C) and the exo-π orbital. It is supposed that some of the electron density of the exo double bond is transferred into the σ*(Cl-C) and is therefore less readily available for reaction with electrophiles. The endo double therefore becomes comparatively more nucleophilic, with a broader electron rich HOMO available for electrophilic addition.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
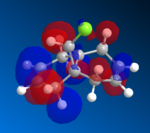
|

|
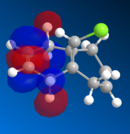
|
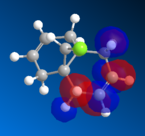
|
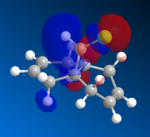
|
Part 2
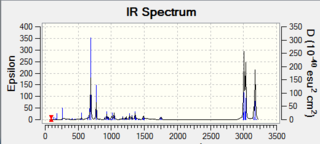
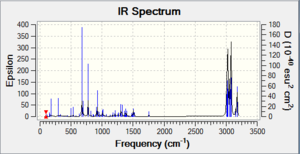
The vibrational frequecies of 12 were calculated using the Gaussian program, running a B3LYP/6-31G(d,p) method. Using the same method, the vibrational frequency of the corresponding monoalkene with the remaining double bond on the same side of the Cl atom was obtained. The vibrational spectra generated from these calculations are reported below, as well as the main vibrations for both molecules. Comparing the data for the two molecules allows to determine the influence of the σ*(Cl-C)/exo-π orbital interaction on the molecule vibrational frequencies.
 |
 |
|---|
Diene:DOI:10042/to-12792
Monoalkene:DOI:10042/to-12793
The suggested σ*(Cl-C)/exo-π orbital interaction should result in a weakening of the C-Cl bond and therefore a decrease in its vibrational frequency. This is indeed observed as the C-Cl frequency goes from 771 cm-1 (diene) to 774 cm-1(monoalkene). Moreover, the transfer of some electron density from the exo π system should result in a weakening of the double bond. This is indeed observed, the endo (1758 cm-1) double bond is stronger than the exo double bond (1737 cm-1). The vibrational frequencies of the two molecules therefore are in good agreement with the orbital interaction explanation provided in Part 1.
| Cl-C Stretch | C=C exo Stretch | C=C endo Stretch |
|---|---|---|
|
|
|
| 770.84 | 1737.05 | 1758.05 |
| Cl-C Stretch | C=C-H bend | C=C endo Stretch | C=C-H aymmetric Stretch | C=C-H Symmetric Stretch |
|---|---|---|---|---|

|
|
|

|

|
| 774.45 | 1425.31 | 1757.45 | 3153.24 | 3175.81 |
Part 3
The electron density of a double bond can be modulated by attaching substituents to it, with electron withdrawing/donating properties. The calculations carried in Part 2 were repeated for a diene molecule bearing sequentially an electron donating (EDG) substituent (=C-SiCH3) and an electron withdrawing (EWG) substituent (=C-CN). One should expect that, as the electron density of the exo double bond is increased (EDG), more electrons will be transferred in the σ*(Cl-C)/exo-π orbital, and should therefore result in a weakening of the C-Cl bond. In the same way, an EWG should strengthen the C-Cl bond. Both effects should be readily observed in the data collected from the Gaussian program, running a B3LYP/6-31G(d,p) method. However, these effect which are purely electronic in nature will not be reproduced using the MM2 method.
| Cl-C Stretch | C=C exo Stretch |
|---|---|
| 765.81 | 1706.25 |
| Cl-C Stretch | C=C exo Stretch |
|---|---|
| 757.04 | 1688.40 |
The results obtained are reported in the two Tables on the side. However, these do not illustrate the expected strengthening and weakening of the C-Cl bonds as straighfowardly as expected. The presence of an EWG weakens the C=C bond; its frequency is reduced from 1737 to 1706 cm-1. However the C-Cl bond, is not strengthen accordingly: the frequency of the C-Cl actually decreases from 771 to 766 cm-1. The difference between the two frequencies is possibly in the range of error of the method used. But it suggests that the C-Cl bond strength is not affected much by the electron density of the C=C bond. Attaching an EDG surprisingly weakens the C=C bond (from 1737 to 1688 cm-1) but weakens the C-Cl bond from 771 to 757 cm-1. This suggest that the excess electron density might not be localised in the π(C=C) orbitals, but instead be transferred into the σ*(C-Cl).
EDG Substituent:DOI:10042/to-12794
EWG Substituent:DOI:10042/to-12795
Conclusion
The reactivity of 12 towards electrophiles was discussed based on results obtained computationally, which successfully explained the regioselectivity of the reaction. Vibrational frequency spectra were generated and analysed in terms of the orbital overlap and put in relation with the reactivity of the molecule.
References
B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
Monosaccharide chemistry: glycosidation
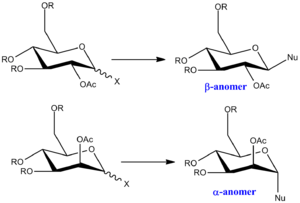
Glycosidation refers to the replacement of group X by a nucleophile Nu. It is found that the two sugars in Figure 6 give different anomers depending on the orientation of the OAc group on the adjacent carbon. This effect comes from the neighbouring-group-participation of the adjacent group, which gives rise to selective attacks from the bottom or from the top face, depending on the orientation of the OAc group. The diastereospecificity of the reaction will be studied using the MM2 and MOPAC/PM6 method.
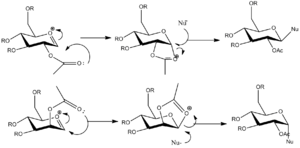
The MM2 method was used in the first place, to obtain a first optimisation for the four different structure of the oxonium ions. These are reported in Table 4. The molecules studied are relatively large ones, and the purely mechanical method that is MM2 is probably not the most appropriate method to use as it does not take into account the electronic interactions in the molecules, which are likely to be important. However, MM2 method does suggest that A and B are more stable than A' and B'.
| Down with acyl above ring plane A' | Down with acyl below ring plane A | Up with acyl above ring plane B | Up with acyl below ring plane B' | |
|---|---|---|---|---|
| Representations | Down above | Down down | Up above | Up down |
| Energies (kcal/mol) | 15.07 | 0.97 | 9.15 | 36.87 |
The MM2 optimised structures were subjected to the MOPAC/PM6 method to further minimise the geometries. The result obtained are reported in Table 6.
| Down with acyl above ring plane | Down with acyl below ring plane | Up with acyl above ring plane | Up with acyl below ring plane | |
|---|---|---|---|---|
| Representations | Down above | Down down | Up above | Up down |
| Energies (kcal/mol) | -85.04 | -91.53 | -91.66 | -77.18 |
The MOPAC/PM6 method heat of formations reflects the stability of the structures (the more negative the heat of formation, the more stable teh structure). In the same way to the MM2 data, it is predicted that that for each pair (AA' BB'), the structures A and B are more stable. One can observe that both structures A and B have very close heats of formation, and that they both feature a very good overlap between the π*(C-O+) and the nO. This is the perfect alignement for the formation of intermediates C and D. Structure A' and B' are less stable than structures A and B, and it is therefore A and B which carry on to form intermediates C and D, in which a five membered-ring intermediate is formed after reaction of the acetal with the oxonium ion.
| MM2 (kcal/mol) | MOPAC/PM6(kcal/mol) | Structures from MOPAC/PM6 | |
|---|---|---|---|
| C | 27.22 | -89.32 | Structure of C |
| C' | 46.12 | -66.85 | Structure of C' |
| D | 37.42 | -74.03 | Structure of D |
| D' | 47.48 | -66.84 | Structure of D' |
The data reported in Table 7 now shows that for each pair (CC' DD'), the structures C and D are more stable. All the relative energies obtained for AA', BB' CC' and DD' can explain the stereoselectivity of the glycosidation in the presence of neighboring- group-effect. Indeed, if the acetal group is pointing down, the carbonyl will be below the plane (as A is more stable than A'), which means that the carbonyl will attack the oxonium ion from below the plane and that the 5-membered ring formed (C) will block the access to the bottom of the plane, which eventually means that the nucleophile will have to attack from the top face. In the same way, if the acetal group is pointing up, the carbonyl group will be above the plane (B more stable than B'), and the nucleophile will therefore be forced to attack from below the plane.
Conclusion
By determining the energies of the different oxonium ions which could form, the regioselectivity of the glycosidation reaction under the neighboring group effect was explained.
References
D. M. Whitfield, T. Nukada, Carbohydr. Res., 2007, 342, 1291. DOI:10.1016/j.carres.2007.03.030
Mini-project: Investigating the regioselectivity of the Baeyer-Villiger reaction
This section acts as the pretext for combining some of the computational methods used in this module 1. A reaction chosen from the primary litterature and reported to give isomeric intermediates will be studied, aiming to characterise and rationalise the observed intermediates.
Discussion
The Baeyer-Villiger reaction reaction is a form of 1,2 sigmatropic shift to O+, taking place when aldehydes or ketones are treated with peracids.
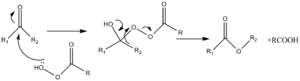
The driving force of these reactions is the exothermic cleavage of the weak O-O bond in the peracid as well as the formation of the C=O bond. The general mechanism for the Baeyer-Villiger reaction is reported in Figure 1. The key step for the regioselective outcome of the reaction is the migration of one of the R groups to the O+. The regioselectivity is indeed determined by the migratory aptitude of the R groups, which itself mirrors the aptitude of the group to stabilise a partial/full positive charge in the transition state.

The synthesis of some analogues of beta-lactam antibiotics reported by J. Marchand-Brynaert et al. in 2004 (DOI:10.1021/jo030377y ) features a Baeyer-Villiger reaction and this particular step will be discussed here. It was found that compound 10, when treated with mCPBA, yielded two products 11 (desired product) & 12 (see Scheme 1). The reaction was performed using various different fucntional groups as the R groups and the ratio of the products obtained experimentally in the crude mixtures are reported in Table 1. The author mentions that these ratio were determined by 1H-NMR.
| R Group | Isomer 11 | Isomer 12 | Energies11 | Energies 12 |
|---|---|---|---|---|
| Ph | 100 % | 0 % | -133(kcal/mol) | -130(kcal/mol) |
| t-Bu | 0 % | 100 % | -190(kcal/mol) | -184(kcal/mol) |
| i-Pr | 52% | 48% | -184(kcal/mol) | -175(kcal/mol) |
| c-Pr | 100% | 0% | -148(kcal/mol) | -136(kcal/mol) |
The first computational calculation performed aimed to determine the relative energies of the regioisomers. This was done using the MOPAC/PM6 geometry optimisation method and are reported in Table 1. These energies, put in relation with the observed ratios of products seem to suggest that the reaction is not under thermodynamic control. As indeed, while isomer 11 b is more stable than 12 b, 11 b is not formed at all. As it has been said, the Baeyer-Villiger reaction's regioselectivity mirrors the migratory aptitude of the R groups, which is a kinetic parameter. From the ratios reported, the migratory aptitudes of the R groups can be put in the following increasing order of migratory aptitude: t-Bu>i-Pr=azetidinyl>Ph=c-Pr.
The author mentions that the the isomers 11 d and 12 d were seperated by chromatography and characterised by 1H-NMR, 13C-NMR and IR spectrocopy.
The spectroscopic characteristics of the two compounds were determined computationally and the data generated was compared to the empirical ones.
| Isomer 11 Computational | Isomer 11 Experimental | Isomer 12 Computational | Isomer 12 Experimental | |
|---|---|---|---|---|
| 1 | 174 | 178 | 162 | 171 |
| 2 | 158 | 167 | 161 | 168 |
| 3 | 36 | 34 | 70 | 70 |
| 4 | 78 | 76 | 59 | 64 |
| 5 | 67 | 65 | 68 | 65 |
| 6 | 63 | 64 | 64 | 50 |
The 13C-NMR spectra of the two molecules were determined using the Gaussian mpw1pw91/6-31g(d,p) method (see Figure 10). The chemical shifts of the terminal methyl groups are not reported in Table 9, as all these appear aroun 20 ppm (+/-2ppm), in both the experimental and computational spectra, are therefore difficult to assign specifically, and do not help in differentiating the two regioisomers.
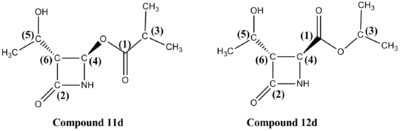
The 13C-NMR chemical shifts obtained experimentally and theoretically are in very good agreement. Two major differences in chemical shifts are observed between the spectra of compounds 11d and 12d for carbon (3) and (4). The different positions of the oxygen atom (close to R group or close to the ring) generate differences in chemical sfits of 20-30 ppm, which makes 13C-NMR a good way of differentiating between the two isomers.
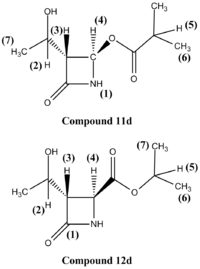
1H-NMR is however clearly the most adapted method for differentiating the two isomers. This is indeed the technique used by the author. The proton chemical shifts were calculated using the Gaussian mpw1pw91/6-31g(d,p)method. The data obtained computationally and experimentally are in less good agreement than the 13C-NMR chemical shift. However, the data is good enough to help in the assignation of a molecule's structure. Chemical shifts, both computationally and empirically obtained, are reported in Table 10. These are referenced to the TMS mPW1PW91/6-31G(d,p) GIAO. The chemical shift of proton (5) provides an unambiguous way of characterising the two isomers.
| Isomer 11 Computational | Isomer 11 Experimental | Isomer 12 Computational | Isomer 12 Experimental | |
|---|---|---|---|---|
| 1 | 6.15 | 7.02 | 5.60 | 6.61 |
| 2 | 4.27 | 4.24 | 4.28 | 4.34 |
| 3 | 3.13 | 3.24 | 3.63 | 3.31 |
| 4 | 5.60 | 5.84 | 4.28 | 4.29 |
| 5 | 2.76 | 2.60 | 5.43 | 5.09 |
| 6 | ~1.30 | 1.19 | ~1.42 | 1.25-1.35 |
| 7 | ~1.40 | 1.35 | ~1.41 | 1.25-1.35 |
An attempt was made to determine the 3J H-H coupling constants using the Janocchio program, which bases its calculations on the Karplus equation. The data obtained from the program is in very poor agreement with the literature values. Very large coupling constants were calculated for J H2-H3 and J H5-H6.
| Experimental | Computational | |
|---|---|---|
| J H3-H4 | 6.3 Hz | 6.7 Hz |
| J H2-H3 | 3.0 | 9.86 Hz |
| J H5-H6 | 6.0 Hz | 1.8 Hz |
11d NMR chemical shifts:DOI:10042/to-12796 12d NMR chemical shifts:DOI:10042/to-12796
Some final computational calculations were performed to determine the vibrational spectra of the two isomers, and to compare the main vibrations to the ones reported in the litterature. Unfortunately, the spectrum of isomer 11d was not obtained. However the frequencies obtained for 12 d are reported in Table 12, along with the literature frequencies.
| 11d Experimental | 12d Experimental | 12d Computational |
|---|---|---|
| 3330 (cm-1) | 3341 (cm-1) | 3789 (cm-1) |
| 1783 (cm-1) | 1749 (cm-1) | 1895 (cm-1) |
| 1740 (cm-1) | 1749 (cm-1) | 1826 (cm-1) |
12d Vibrational Frequencies:DOI:10042/to-12786
The frequencies obtained from the computational method are in poor agreement with the literature ones. Moreover, vibrational frequency does not seem to be a method which allows to differentiate the isomers very efficiently, as differences in frequencies are not important enough.
Conclusion
Both 13C and 1H NMR data were obtained for the two isomers, compared to the literature values and appear to be in very good agreement with the laters. It was found that both spectroscopic techniques should allow to differentiate the two isomers, but 1H-NMR was preferred by the author, probably because of its greater sensitivity. An attempt was made to calculate coupling constants from the Janocchio program, but the result obtained were very poor and not much information could be taken from them. Some vivrational frequencies were calculated, but the frequencies obtained did not agree with the literature ones. Moreover, IR spectroscopy does not provide very valuable information for the differentiation of the two molecules. Overall, it however appeared that computational prediction of spectroscopic properties can be of great help for the characterisation and assignation of molecular structure.
