Rep:Mod:jg2016-hunt
Revision Section
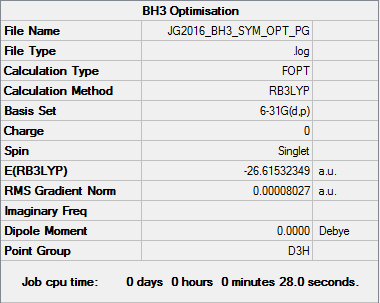
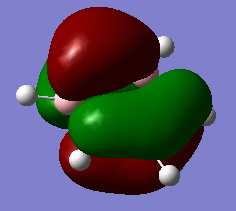
BH3
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Low frequencies --- -1.1800 -1.0028 -0.0055 4.1927 11.0182 11.0637 Low frequencies --- 1162.9912 1213.1792 1213.1819
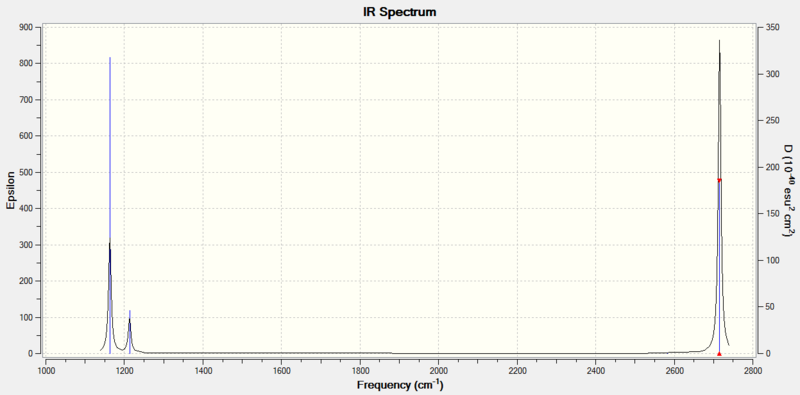
| Frequency/cm-1 | Intensity | Symmetry | IR Active? | Type of Vibration |
|---|---|---|---|---|
| 1164 | 93 | A2" | Yes | Out of plane Bend |
| 1214 | 14 | E' | Slightly | Bend |
| 1214 | 14 | E' | Slightly | Asymmetric in plane Bend |
| 2580 | 0 | A1' | No | Symmetric Stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch in plane |
| 2713 | 126 | E' | Yes | Asymmetric stretch in plane |
There are less than 6 peaks in the IR spectrum of BH3 even though there are 6 distinct vibrations because the A1' vibration at 2580 cm-1 doesn't produce a change in dipole moment and so is IR inactive and will not produce a peak at all. In addition, there are 2 vibrations which have the exact same symmetry and energy at 1214 cm-1 and so their individual peaks will superimpose to only show 1 peak. The exact same situation is happening with the 2 vibrations at 2713 cm-1 so these 2 vibrations will be superimposed and only show 1 peak. This explains why only 3 peaks are seen in this spectrum.
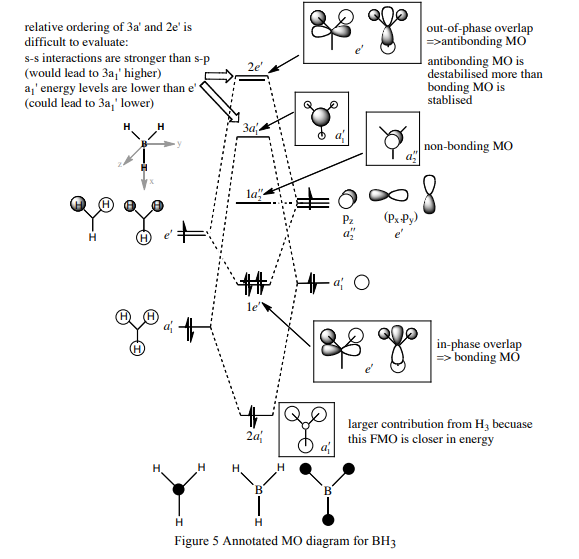
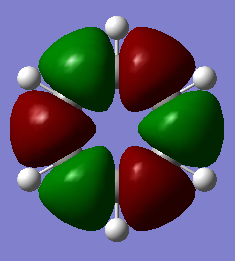
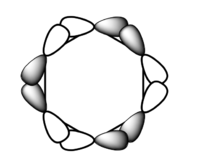
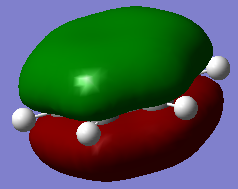
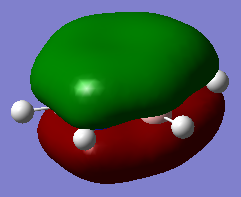
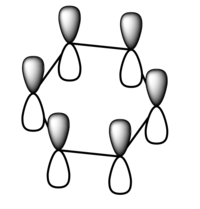
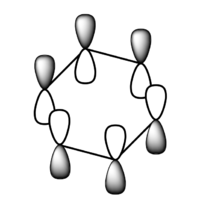
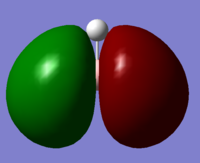
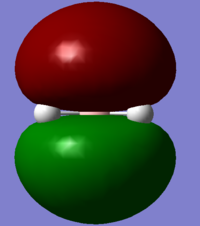
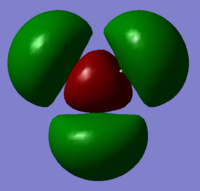
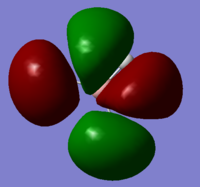
| 1a1' | 2a1' | 1e' | 1e' | 1a2" | 3a2' | 2e' | 2e' |
|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
|
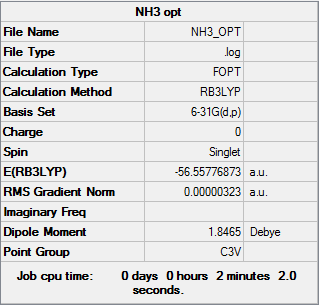
NH3
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
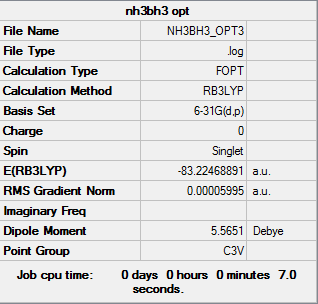
NH3BH3
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000531 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Media:NH3BH3 FREQ 2 jg2016.LOG
Low frequencies --- -0.0251 -0.0031 0.0007 17.1240 17.1263 37.1338 Low frequencies --- 265.7821 632.2034 639.3485
Association Energy
E(NH3)=-56.55777 A.U.
E(BH3)=-26.61532 A.U.
E(NH3BH3)=-83.22469 A.U.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]=0.0516 A.U.=135 kJ mol-1
This means that the B-N dative bond is quite weak because the bond C-C dissociation energy is 731.5 kJ mol-1. [2]
Smf115 (talk) 15:54, 28 May 2018 (BST)Correct calculations and accuracy of the final reported energies, however, the C-C bond dissociation energy is actually 348 kJ/mol (Atkins Physical Chem 8th edition DATA section Table 11.3b) so be careful what values are used for the comparison.
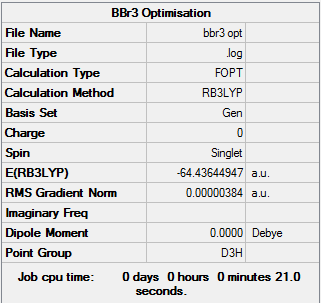
BBr3
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000024 0.001200 YES
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
Investigating Aromaticity
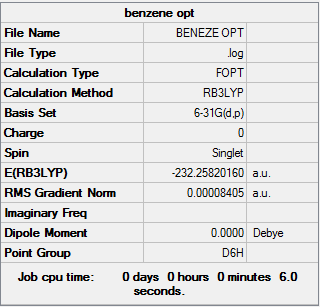
Benzene
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000077 0.000300 YES Maximum Displacement 0.000824 0.001800 YES RMS Displacement 0.000289 0.001200 YES
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0055 -0.0055 -0.0009 Low frequencies --- 414.1239 414.1239 620.9400
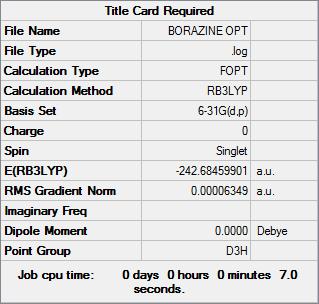
Borazine
Item Value Threshold Converged? Maximum Force 0.000084 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000252 0.001800 YES RMS Displacement 0.000074 0.001200 YES
Media:BORAZINE FREQ jg2016.LOG
Low frequencies --- -0.0010 -0.0010 -0.0010 3.5555 4.4272 6.8943 Low frequencies --- 289.7109 289.7845 404.4187
Analysis
Charge Distribution
 |
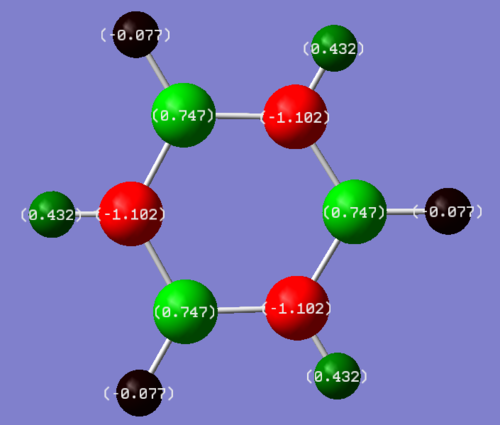 |
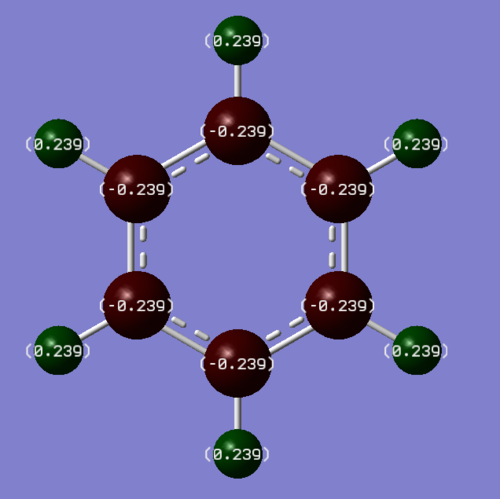
In benzene all the atoms involved in the aromaticty (the carbons) have the same, slight negative charge of -0.239 and therefore all the hydrogens also have the same positive 0.239 charge. In borazine the boron atoms have a +0.747 charge while all the nitrogen atoms have a -1.102 charge. The hydrogen atoms bonded to the boron atoms have a -0.077 charge and the hydrogen atoms bonded to the nitrogen atoms have a +0.432 charge.
Carbon and hydrogen have similar electronegativities (2.5 and 2.1) and so there is minimal charge separation between the carbon and hydrogen bond in benzene. Nitrogen is more electronegative (3.0) than Boron (2.0)[2] and so in the aromatic ring of borazine there will be a build up of electron density at the nitrogen atoms and a deficiency of electrons at the boron atoms. Hydrogen is less electronegative than nitrogen and so the hydrogens bonded to nitrogen will have a very positive charge. Boron and hydrogen have very similar electronegativities but the boron atoms in aromatic ring are partially positive so the hydrogens attached to boron in borazine have a very small negative charge.
Smf115 (talk) 13:12, 1 June 2018 (BST)Good use of electronegativities to explain the charge distribution and the sam colour range is used across both molecules which is correct. To improve, consider other factors such as symmetry and the net charge of the molecule.
Molecular Orbitals
Smf115 (talk) 13:15, 1 June 2018 (BST)Nice MO comparison with comparable MOs selected and the contributing AOs have been considered with the nice inclusion of the LCAO diagrams. However, the evaluation of the bonding/anti-bonding character of the orbitals isn't always correct and it would have been nice to see a larger range across the MOs chosen.
Aromaticty
Aromaticty in its most basic sense describes a molecule with a delocalised cyclic pi electron system. Traditionally a molecule was described as aromatic if it had 4n+2 pi electrons and was cyclic and planar. This produced an aromatic stabilisation energy which made these molecules more stable when compared to its theoretical non aromatic structure, and had bond lengths in between single and double bonds. This simple description of aromaticty doesn't account for the molecules which exhibit aromaticity even though they aren't planar and undermines the contribution of sigma bonding to the aromatic character of some molecules.[3]
Originally delocalisation of electrons around an aromatic molecule was thought to be due to pz orbital overlap but through calculations using molecular orbital theory it can be seen that other atomic orbitals will combine to form molecular orbitals which delocalise electrons around the molecule. For example, MO 14 for benzene shown above is not formed from the linear combination of pz orbitals above and below the plane of the molecules but px orbitals in the plane of the molecule. This produces a delocalised sigma framework which will be equally as responsible for stabilising aromatic compounds as the pi bonding system. This means that stating overlapping pz orbitals as a description for aromaticity is inaccurate because many other atomic orbitals in each atom will be responsible for producing MOs which delocalise electrons around the molecule and producing the stabilising effect attributed to aromaticity.
Smf115 (talk) 13:16, 1 June 2018 (BST)A good wiki report which lacks some information at times but is a nice attempt overall.
References
- ↑ Hunt.P (2018). Molecular Orbitals in Inorganic Chemistry. Tutorial after Lecture 4 Notes. [pdf] Retrieved from http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf.
- ↑ 2.0 2.1 Sanderson, R. (1975). Interrelation of bond dissociation energies and contributing bond energies. Journal of the American Chemical Society, 97(6), pp.1367-1372.
- ↑ Palusiak, M. and Krygowski, T. (2007). Application of AIM Parameters at Ring Critical Points for Estimation of π-Electron Delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings. Chemistry - A European Journal, 13(28), pp.7996-8006.