Rep:Mod:jfa1995
Introduction
Potential Energy Surface
A potential energy surface is a molecule's total energy as a function of the atoms relative positions, calculated by use of the Schrodinger equation. For a triatomic molecule this can be plotted in 3D (using the 3N-6 rule) and thus the energy can be seen as a "surface" dependent on the positions of the atoms. The number of coordinates obviously increase with bigger systems.[1]
Reaction path and transition state
The minimum potential energy path, the reaction coordinate, between any two compounds will be where the gradient is zero in some direction (as it must be the lowest energy possible along that path) and will once cross a saddle point which marks the maximum potential energy along the reaction coordinate. This is where the transition state is found. Likewise, any stable compound can be found at a local minimum as any perturbation would cause a raise in energy.[1] Nf710 (talk) 19:47, 16 November 2016 (UTC)(2 issues here, a minimum on the PES is where the gradient is zero in all dimensions (degrees of freedom) and the curvature second derivative is positive in all directions. A TS is a maximum but only in 1 degree of freedoma and this is indicated by one imaginary frequency. In 2 dimensions this can be visulised as a saddle point.)
Kinetics and thermodynamics
The energy barrier, the difference between the reactant and the transition state, is independent of the actual energy difference between the starting material and the product, and is thus the kinetic barrier. Thermodynamics, however, is dependent simply on the actual energy level of the compounds.
Exercise 1
Reaction scheme

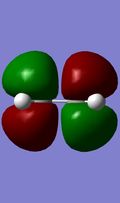
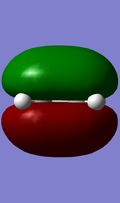
HOMO/LUMO interactions of reactants

The above MO diagram shows the FO combinations. The FO's can be seen below, and the combinations in the transition state can be seen in the Jmol below, or for more accurate images please view the content on this link. In this example the FO's all have centers of conversion, thus are either gerade or ungerade. It's seen in the images (also illustrated in the MO diagram) that only the FO's of the same symmetry overlap. That is explained by the fact that the net orbital overlap between a gerade and ungerade FO is 0, hence it is non-bonding.
| Reactants | |||
|---|---|---|---|
 |
 |
 |
 |
Nf710 (talk) 20:01, 16 November 2016 (UTC) You need to set the frame correctly in the jmol
C-C bond lengths
Transition state vibrations and bond formation
As seen below (activate vibrations) the imaginary vibration wavenumber, which follows the reaction path, implies a synchronous bond formation. In comparison the lowest positive wavenumber only represents a "wiggly" motion of the two reactants individually.
Nf710 (talk) 20:07, 16 November 2016 (UTC) Abit brief you could have said more
Exercixe 2
Reaction scheme
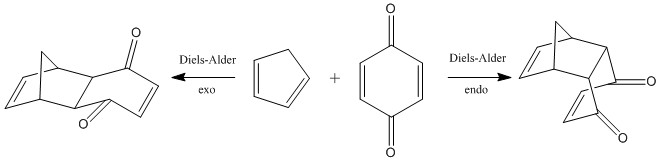
Molecular orbitals of the exo/endo Diels-Alder reaction
The reactant FO's can be seen below, and the combinations in the transition state can be seen in the Jmol below, or for more accurate images please view the content on this link.
| Reactants | |||
|---|---|---|---|
 |
 |
 |
 |
Exo Diels-Alder transition state
Endo Diels-Alder transition state
(You've chosen the wrong orbitals for the HOMOs and LUMOs. It looks like you've chosen HOMOs to have an orbital energy of <0eV. However you should be looking at their occupancy (orbitals 45-48) Tam10 (talk) 12:06, 9 November 2016 (UTC))
Normally a Dieals-Alder reaction is follows an attack of an electron rich, lower-in-energy, diene onto an electron poor dienophile. There are cases of inverse Diels-Alder reactions where the dienophile is more electron rich, however according to the calculated orbital energy levels - as seen in the table below - the HOMO of the diene is higher in energy than the HOMO of the dienophile, hence the diene is more electron rich and it is a normal Diels-Alder reaction.
Energy levels of FO's
| FO | Cyclopentadiene | Benzoquinone |
|---|---|---|
| LUMO+1 | 0.08991 | 0.02648 |
| LUMO | -0.00984 | -0.12991 |
| HOMO | -0.21154 | -0.27056 |
| HOMO-1 | -0.29351 | -0.27882 |
NOTE: There's an error in this table, and the actual HOMO of benzoquinone is -0.12991. Thus, by the same reasoning as above, this must be an inverse Diels-Alder.
Transition state and reaction energies
Total energies in room temperature (kJ/mol)
| Benzoquinone | Cyclopentadiene | Exo TS | Exo product | Endo TS | Endo product |
|---|---|---|---|---|---|
| 14.63 | 293.26 | 475.77 | 281.04 | 473.25 | 283.78 |
Thermodynamic/kinetic values (kJ/mol)
| Exo activation energy | Exo reaction energy | Endo activation energy | Endo reaction energy |
|---|---|---|---|
| 167.88 | -26.85 | 165.36 | -24.11 |
The reason why the endo is the kinetically favourable product (lesser activation energy, so easier to pass the energy barrier) is likely because of secondary orbital interactions, where the AO's of the two carbons adjacent to the two double bonds of the cyclopentadiene overlap with those of the carbons adjacent to the oxygen of the benzoquinone.[2] This can be seen in the Jmol above, for HOMO-1.
The exo product is the thermally more stable (bigger overall loss in energy), probably because of less steric repulsion.
Nf710 (talk) 20:37, 16 November 2016 (UTC) I tried to figure out what you have done with your energies. They are very out though and you have come to the wrong conclusion.
Nf710 (talk) 12:12, 18 November 2016 (UTC) It does however look like yo have got the correct TS
Exercise 3
Reaction scheme
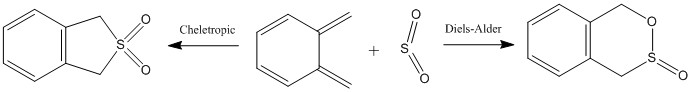
Transition state and reaction energies
Total energies in room temperature (kJ/mol)
| Sulphur dioxide | Xylylene | D-A exo TS | D-A exo product | D-A endo TS | D-A endo product | Cheletropic TS | Cheletropic product |
|---|---|---|---|---|---|---|---|
| -311.42 | 470.63 | 241.74 | 56.33 | 237.76 | 56.99 | 260.10 | 0.000 |
Thermodynamic/kinetic values (kJ/mol)
| D-A exo activation energy | D-A exo reaction energy | D-A endo activation energy | D-A endo reaction energy | Cheletropic activation energy | Cheletropic reaction energy |
|---|---|---|---|---|---|
| 82.52 | -102.88 | 78.55 | -102.22 | 100.88 | -159.21 |
According to this data the cheletropic reaction is the thermodynamically most favourable reaction, but kinetically least favourable. Between the two Diels-Alder reactions the exo is slightly more favourable thermodynamically, and the endo clearly favourable kinetically.
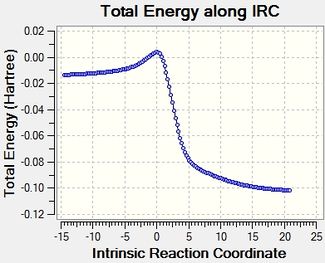
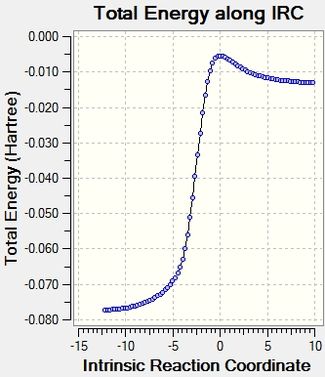
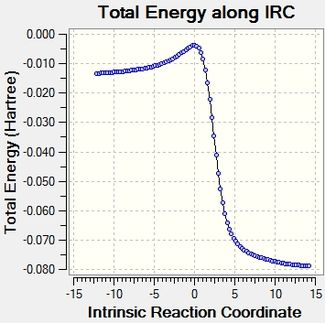
Reaction profile and visualised reaction mechanism
The reaction profiles cohere well with the calculated energies, and it is illustrated clearly how the reaction energy is actually much bigger than the activation energy.
(You need to have an overlay of each of the reactions to compare the barriers and reaction energies Tam10 (talk) 12:06, 9 November 2016 (UTC))
References
<references>