Rep:Mod:jem3ii
The Diels Alder Cycloaddtion
Firstly, cis butadiene was built in Gaussview and C-C bond lengths and angles were set using data from a previous molecular mechanics study.[1] This was "cleaned" then optimised to a minimum using HF/3-21G. The energy gradient and summary suggested that this had been successful. A frequency analysis was done using the same methods and no negative frequencies were found.

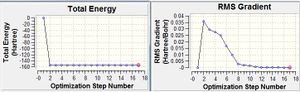
The thermochemistry data is shown below:
Zero-point correction= 0.118498 (Hartree/Particle) Thermal correction to Energy= 0.122530 Thermal correction to Enthalpy= 0.123474 Thermal correction to Gibbs Free Energy= 0.092955 Sum of electronic and zero-point Energies= -155.112862 Sum of electronic and thermal Energies= -155.108830 Sum of electronic and thermal Enthalpies= -155.107886 Sum of electronic and thermal Free Energies= -155.138405
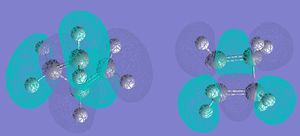
Next, the Mos were visualised. The HOMO was asymmetrical with respect to the reflection plane of the reaction the mole, and the LUMO was symmetric.
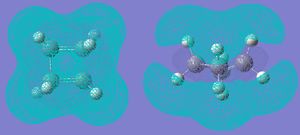
Next, the optimised structure and ethene were drawn, and a TS guessed. The TS was guessed by modifying bicyclo[2,2,2]octane, deleting 2 carbons and changing or deleting other bonds, then TS(Berny)calculation was used.
TS(Berny) Optimisation: Item Value Threshold Converged? Maximum Force 0.000022 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000421 0.001800 YES RMS Displacement 0.000073 0.001200 YES Predicted change in Energy=-5.010461D-09 Optimization completed. -- Stationary point found.
The output gave 1 imaginary frequency at -554cm-1. Visualising this frequency suggested that both bonds formed at the same time. The lowest positive frequency was 165cm-1. This would have suggested asynchronous bond formation.
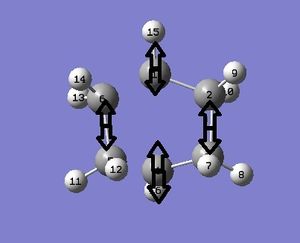
The thermochemistry data is also shown below:
Zero-point correction= 0.152697 (Hartree/Particle) Thermal correction to Energy= 0.157712 Thermal correction to Enthalpy= 0.158656 Thermal correction to Gibbs Free Energy= 0.124359 Sum of electronic and zero-point Energies= -231.388077 Sum of electronic and thermal Energies= -231.383062 Sum of electronic and thermal Enthalpies= -231.382118 Sum of electronic and thermal Free Energies= -231.416414
The Mos were then visualised. Both were found to have a sigma v plane of symmetry relative to the plane of the forming ring, and a C2 axis lying along this plane.
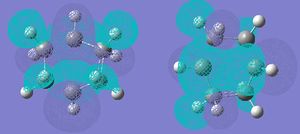

The above calculations were re-done using a higher level (B3LYP/6-31G(d) - outputs were checked as before)to give the following results:
| C-C bond forming length/A | C-C (from ethene) bond length/A | C=C bond forming length/A | C-C (from butadiene) lengths/A | Butadiene dihedral angle/o | |
|---|---|---|---|---|---|
| 1st optimisation | 1.5 | 1.5 | 1.5 | 1.5 | 29.3 |
| 2nd optimisation | 1.5 | 1.6 | 1.6 | 1.5 | 22.2 |
The dihedral angle was the main difference in geometry. The Mos were also visualised using the more accurate basis set, however there were no significant changes. The imaginary frequency did however change to 311cm-1, and the lowest positive frequency to 116cm-1. The imaginary frequency appear the same, but the positive one was less conclusively asynchronous at the higher level.
A normal C-C bond has length 1.54A, and C=C 1.36A. The van der Waals radius of carbon is 1.70A (Webelements). This means that the distance between the carbons about to form a new bond in the TS is less than that of the sum of the van der Waals radii.
The thermochemistry data did change considerably:
Zero-point correction= 0.141771 (Hartree/Particle) Thermal correction to Energy= 0.147361 Thermal correction to Enthalpy= 0.148305 Thermal correction to Gibbs Free Energy= 0.112772 Sum of electronic and zero-point Energies= -234.369822 Sum of electronic and thermal Energies= -234.364233 Sum of electronic and thermal Enthalpies= -234.363289 Sum of electronic and thermal Free Energies= -234.398822
The HOMO of ethene is symmetric with respect to the reflection plane, whereas its LUMO is antisymmetric. A reaction is allowed if a HOMO-LUMO interaction between the reactants is possible. Since a + a --> a, s + s --> and s + a --> a, the product must be antisymmetric with respect to the reflection plane. This can be seen in the TS:
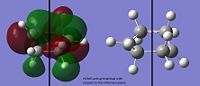
This is confirmed by looking at the MOs of the cis1,3-butadiene. The HOMO reacts with the pi antibonding orbital of the ethene to give the TS. The HOMO/LUMO interaction and the fact that there is good orbital overlap means that the reaction is allowed.
Cyclohexa-1,3-diene and Maleic Anhydride
Exo TS
The exo TS was optimised and thermochemistry data found:
Zero-point correction= 0.195859 (Hartree/Particle) Thermal correction to Energy= 0.204620 Thermal correction to Enthalpy= 0.205564 Thermal correction to Gibbs Free Energy= 0.161353 Sum of electronic and zero-point Energies= -605.383190 Sum of electronic and thermal Energies= -605.374429 Sum of electronic and thermal Enthalpies= -605.373485 Sum of electronic and thermal Free Energies= -605.417696

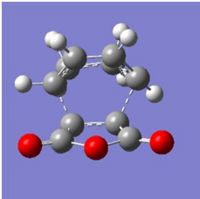

Bond lengths were investigated, and will be compared with the endo TS (see later).
The TS HOMO was found:
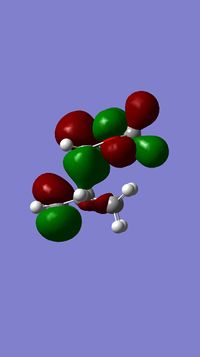
....................Bond forming lengths 1.69A, 1.54A. The HOMO is therefore not totally symmetrical and doesn’t have a perfect C2 axis or sigma v plane. C-C distances in maleic anhydride part: (C1-2 and C3-4)1.51 and 1.52 C13-14: 1.49 C14-15: 1.32 C15-10: 1.53 C10-11: 1.69 C11-12: 1.55 C12-13: 1.54
(CO)-O-(CO): 112.7o......................... Endo: the first QST3 optimisation failed:
The failed structure was then optimised again:
- ↑ D. Guay,Dept of Chemistry, University of Maine, Orono, ME 04469{{|http://chemistry.umeche.maine.edu/Modeling/donmolmech.html }}
