Rep:Mod:jem2ii
Jenifer Mizen: Mini-Project on cyclic germanium compounds
Carbon-less analogues of the cyclopropenium ion have been synthesised using germanium to give a Huckel aromatic 2 pi electron tricyclic compound.[1] This project aims to use computational methods to investigate the aromaticity of the cyclogermanium cation, and to compare this with the double bond containing starting material. The reaction of the cyclotrigermenium to form a chlorinated product will also be investigated.[2]
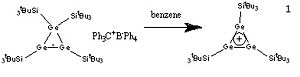
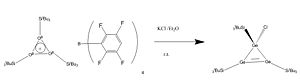
Firstly,(SiH3)4Ge3, (SiH3)3Ge3+ and (SiH3)3Ge3Cl were all optimised, then a frequency analysis done to check that the minima were found. Next the MOs were found to check if the cyclopropenium analogue did indeed have a diffuse cloud of pi electrons above and below the plane of its ring.
Optimisation
Each compound was drawn in ChemDraw, and its energy optimised using a quick MM2 method. This was then opened in Gaussview and an optimisation done via SCAN. The summaries, energy gradients over optimisation steps, and the real output files were checked. The data is shown below.
(SiH3)4Ge3
http://hdl.handle.net/10042/to-5999
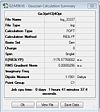

Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.001794 0.001800 YES RMS Displacement 0.000423 0.001200 YES Predicted change in Energy=-5.363609D-09 Optimization completed. -- Stationary point found.
(SiH3)3Ge3+
http://hdl.handle.net/10042/to-6000
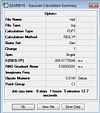

Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.001350 0.001800 YES RMS Displacement 0.000348 0.001200 YES Predicted change in Energy=-4.023038D-09 Optimization completed. -- Stationary point found.
(SiH3)3Ge3Cl
http://hdl.handle.net/10042/to-6002
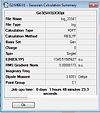

Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000761 0.001800 YES RMS Displacement 0.000225 0.001200 YES Predicted change in Energy=-9.349906D-10 Optimization completed. -- Stationary point found.
All of the results show a small energy gradient, and the output files show that the calculations converged. This suggests that the optimisations worked.
Frequency Analysis
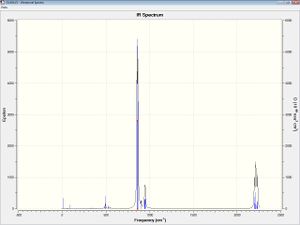
Next, a frequency analysis was done to check that the correct minima were found. The summary files and IR of each other compounds are shown below, as are the most important frequencies from the output file.
(SiH3)4Ge3
http://hdl.handle.net/10042/to-6005
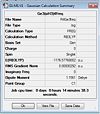
Low frequencies --- -4.3334 -3.3719 -1.2177 -0.0011 0.0011 0.0024 Low frequencies --- 13.9119 33.4360 43.8202 Frequencies -- 13.8015 33.3995 43.8075
(SiH3)3Ge3+
http://hdl.handle.net/10042/to-6009
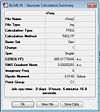
Low frequencies --- -4.3198 -2.3293 -0.0018 -0.0018 -0.0017 1.5929 Low frequencies --- 5.4173 6.6054 17.2145 Frequencies -- 3.1450 5.7683 17.1228
(SiH3)3Ge3Cl
http://hdl.handle.net/10042/to-6007
Low frequencies --- -2.9549 0.0006 0.0008 0.0011 2.5907 4.6629 Low frequencies --- 20.4114 29.9269 37.5817 Frequencies -- 20.4090 29.9108 37.5714
In the output files, the low frequencies were all lower than the real frequencies, and there were no negative IR frequencies, suggesting that the minima had been found in the previous optimisation. The optimised structures are therefore shown below:
Note on the IR spectra: Most of the peaks are due to various motions of the hydrogens in the SiH3 ligands, however there are a couple of noteworthy peaks. 106cm-1 for the halogenated product is due to the Cl, as is 323cm-1. Also, at around 200 and 335cm-1 for the cation, there are stretches due to the deformation of the Ge3 ring. These are found at higher frequencies, with very small intensities, in the chlorinated product (346 and 403cm-1) and not at all in the starting material.
MO and NBO Analysis

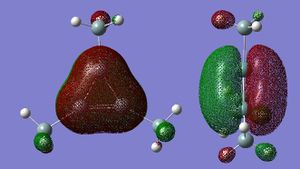

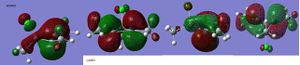
(SiH3)4Ge3
http://hdl.handle.net/10042/to-6011
The NBO analysis output states that, for each Ge-Ge bond, there was approximately a 50% contribution from each Ge. The s and p contributions were as follows:
1. 1s 22.44%, p 77.56%
2. 1s 30.47%, p 69.53%
3. 1s 10.14%, p 89.86%
These hybridisations of the orbitals are pictorially shown on the HOMO MO diagram.
(SiH3)3Ge3+
http://hdl.handle.net/10042/to-6012
1. 1s 27.82%, p 72.18%
2. 1s 27.80%, p 72.20%
3. 2s 27.74%, p 72.26%
This time the s and p characteristic of each bond is almost identical.
(SiH3)3Ge3Cl
http://hdl.handle.net/10042/to-6013
1. 1s 21.81%, p 78.19%
2. 1s 24.06%, p 75.94%
3. 2s 25.44%, p 74.56%
These values are more regular than those for the starting material, but differ from the cyclopropenium ion analogue.

The charges on each Ge were also found for the three species.
| Charge 1 | Charge 2 | Charge 3 | |
|---|---|---|---|
| Starting material | -0.116 | -0.003 | -0.002 |
| Cyclopropenium cation analogue | 0.128 | 0.127 | 0.127 |
| Chlorinated product | 0.345 | -0.036 | -0.049 |
Comparison
| Si-Ge bond length/A | Ge-Ge bond length/A | Bond angles /o | |
|---|---|---|---|
| Starting material | 2.42, 2.42, 2.42 | 2.55, 2.55, 2.33 | 54.5, 62.7, 62.8 |
| Cyclopropenium cation analogue | 2.44, 2.44, 2.44 | 2.40, 2.41, 2.41 | 60.0, 60.0, 60.0 |
| Chlorinated product | 2.41, 2.42, 2.42 | 2.33, 2.42, 2.51 | 55.7, 61.7, 62.6 |
The main parameter to compare was the Ge-Ge bond length. The Ge=Ge bond length was, as expected, shorter than the Ge-Ge (2.33 and 2.55A respectively) in the starting material. Literature values were 2.239A and 2.522A.
On cation formation, the previous Ge-Ge length changed to 2.41 (or 2.40)A, which is part way between the double and single bond length. The Si-Ge bond length increased relative to that in the starting material. Literature values were 2.325, 2.321 and 2.333A for a cation stabilised by a BPh4- anion.
On chlorination, the Ge=Ge bond length returned to its initial 2.33, but the other two Ge-Ge bond lengths were found to be shorter than in the starting material and longer than in the cyclopropenium analogue. Literature values were 2.2723A, 2.4293A and 2.4225A. This also gives three different lengths, with the one opposite the Cl as the shortest. Values for bond angles were 55.85 (on the Ge bonded to Cl),61.92 and 62.23o. These are very close to the ones computed.
The bond angles followed similar trends to the bond lengths - those in the starting material show a definite isocelese nature for the Ge3 triange, the cyclopropenium ion is equilateral, and the chlorinated product tends more towards the form of the starting material.
The change to equal bond lengths and angles in the cyclotrigermenium cation strongly suggests that it has become aromatic (from a non-aromatic starting material). The planarity of the structure also supports this.
Looking at the NBO analysis suggests the same - the charge is delocalised over the three Ges in the cation. The MO diagrams provide further evidence. A delocalised pi cloud appears either side of the plane of the ring in the HOMO of the cation, whereas this was not seen in the starting material.
However the starting material did contain some interesting overlap where the HOMO appears to be composed of three pi type Ge orbitals interacting with each other to form just two bigger lobes (one - and one +).
The HOMO for the chlorinated product shows delocalisation over the Ge=Ge. One lobe of this pi cloud interacts with the SiH3 group on the third Ge. This gives a larger s charater to the Ge bonded to the Cl when compared to the starting material, as can be seen from the NBO analysis, suggesting that replacing an SiH3 with a Cl confirs extra stability to the molecule. This suggestion is supported by the fact that the average bond length is shorter for the chlorinated compound.
The literature says that there is a σ*−π interaction between the pi of the Ge=Ge and the antibonding sigma orbital of the Ge-Cl. This could be imagined if the lobes surrounding the Cl were reversed.
Conclusion
The tricyclicgermenium ion does display aromatic properties when considering its geometry and MOs. This could be synthesised from a non-aromatic starting material. In the halogenated product, there is interesting orbital overlap due to the Cl-Ge interacting with the Ge=Ge, which makes this product a little different in geometry to the starting material.
References
- ↑ A. Sekiguchi, M. Tsukamoto and M. Ichinohe, Science, 1997, 275, 60. DOI:10.1126/science.275.5296.60
- ↑ A. Sekiguchi, Y. Ishida, N. Fukaya and M. Ichinohe, J. Am. Chem. Soc. , 2002, 124, 1158. DOI:10.1021/ja012216m
