Rep:Mod:jem
Molecular Mechanics
Molecular mechanics computational methods avoid solving the Schrodinger wave equation for the molecule by considering individual bond properties. This reduces computation time, and in many cases (i.e. where bonds and interactions in the molecule are simple and diatomic-like) it yields good results. The methods assume that the total energy of the molecule is made up of contributions from diatomic bond stretches, triatomic angle deformations, bond torsions, non-bonded van der Waals repulsions and electrostatic interactions of individual bond dipoles. It uses Classical methods, for example Hooke's law for the first two parameters listed, and presumes that the contributions are all additive and do not interact. This deviates from reality when secondary orbital interactions become important, for example in hyperconjugated molecules, and anywhere where MO theory is needed to explain molecular properties.
The programs used in the project are ChemBio3D, and Gaussian for the DFT methods used later on. The Allinger MM2 model was used in ChemBio3D and it should be noted that the energies calculated are not related to any measureable thermodynamic measurement, and should only be used for comparative purposes between isomers.
More about Density Functional Theory and Gaussian can be found here: http://www.gaussian.com/g_tech/g_ur/k_dft.htm.
Hydrogenation of Cyclopentadiene
Cyclopentadiene may dimerise to form either an exo (1) or an endo (2) dimer. MM2 calculations were run to investigate this reaction. It was found that the exo dimer is lower in energy, so if the reaction were thermodynamically controlled, this would be the product, however it is known from experiment that the endo dimer is formed, so the reaction must be kinetically controlled.
Energy of starting materials:
Exo 1: 31.9 kcal/mol
Endo 2: 34.0 kcal/mol

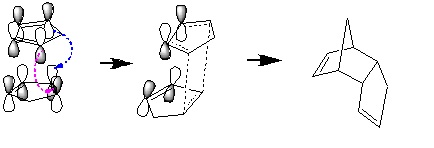
The formation of the endo product is due to favourable orbital overlap in the endo transition state:
Hydrogenation of the endo dimer could give one of two products (3 and 4). The energies of both were calculated:
| Stretch | Bend | Stretch-bend | Torsion | Van der Waals | Dipole/dipole | Total energy (kcal/mol) | |
|---|---|---|---|---|---|---|---|
| 3 | 1.2 | 19.0 | -0.8 | 12.1 | 4.2 | 0.2 | 35.9 |
| 4 | 1.1 | 14.5 | -0.6 | 12.5 | 3.5 | 0.1 | 31.2 |
4 is therefore the thermodynamic product. This is due to the higher energy of the bend in 3 and to the van der Waals stabilisation in 4.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
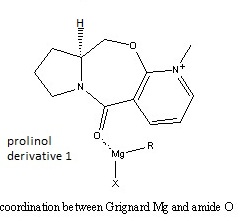
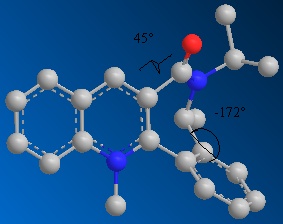
On reacting MeMgI with the prolinol derivative 1, the methyl adds para to the positive nitrogen, on the opposite face to the ring compared to the hydrogen shown.
A low and a high energy conformation of 1 are shown:
The strain in the ring is much greater for the high energy structure. Most initial conformations of 1 were minimised to the first, low energy structure by the MM2 optimisation method. (ChemBio3D displayed an error message when the MgMeI was included and the geometry optimisation run: "no atom type was assigned to te selected atom". This suggests that either it cannot optimise two molecules at the same type, or that it cannot process the metal atom.)
The positive charge on the nitrogen has resonance structures with the charge moved to the ortho and the para positions, so the nucleophile will attack either ortho or para to the nitrogen. However the coordination of the amide oxygen to the magnesium of the Grignard reagent directs para attack.[1]
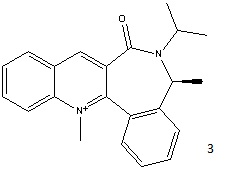
On addition of aniline to 3, the PhNH group adds with the same stereochemistry relative to the ring as the Me group ("forwards"). The lowest energy form of 3 is shown below:
There are also different arrangements of the 6-membered aromatic ring and the methyl, for example:
The different configurations are due to atropisomerism (i.e. restricted rotation about a single bond).
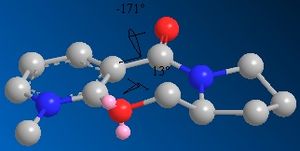
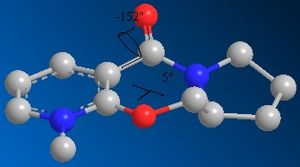
The product sterochemistry can be explained by the fact that an NHR- group will add on the opposite face to the C=O of the lactam [2]. The dihedral angle between the C=O and the 6-membered ring containing the N+ was found to be small, but negative (-20 degrees) for the lowest energy conformation, and small but positive (+18 degrees) for the higher energy one. The 6 membered ring opposite the C=O is bent in the same direction, also encouraging nucleophilic addition on the opposite face.
The ChemBio3D version 12.0 is known to contain a bug which affects molecular mechanics calculations if N+ is present. The calculations were therefore redone using the MOPAC molecular orbital PM6 method. The dihedral angles shown both change on changing the calculation method.
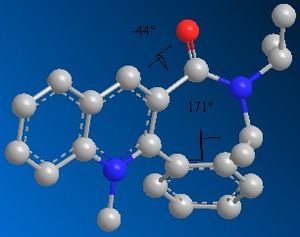
For molecule 7, on redoing the geometry optimisation, the dihedral angle more than doubled to -44 degrees for the lowest energy conformation, and the heat of formation found was 156.2 kcal/mol. Likewise, for the higher energy conformation, the angle changed to 45 degrees, and, surprisingly (due to the previous MM2 calculations!), both heats of formation were very similar, with the second being 157.4 kcal/mol. This was not the only dihedral angle to change. The angling of the 6-membered ring with respect to the 7-membered one changed considerably.
The product formed is known as a masked amide, and the starting material an amide transfering reagent, as the amide can now be released and added on to an ester, and the starting material regenerated.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
An example of atropisomerism is found in the taxol intermediate shown. Two conformations are possible - 1, with the large ring in a twist-boat, and 2 with the ring in a chair shape.
Taxol intermediate 1 |
Taxol intermediate 2 |
The energies of 1 and 2 were compared. Reactions involving breaking of the double bond are known to be very slow. This was also investigated by running the MM2 calculations on the hydrogenated forms of 1 and 2, and studying the energy changes.
| Stretch | Bend | Stretch-bend | Torsion | Van der Waals | Dipole/dipole | Total energy (kcal/mol) | |
|---|---|---|---|---|---|---|---|
| Taxol intermediate 1 | 2.9 | 15.1 | 0.3 | 22.6 | 15.1 | 0.0 | 56.0 |
| Taxol intermediate 2 | 0.3 | 20.5 | -0.1 | 20.2 | 16.9 | 0.1 | 58.6 |
| Hydrogenated 1 | 1.1 | 25.2 | -0.1 | 26.9 | 23.5 | 0.0 | 76.7 |
| Hydrogenated 2 | 1.6 | 37.7 | 0.2 | 22.0 | 29.6 | 0.0 | 91.0 |
On hydrogenating the double bond, the energy increases, suggesting that this bond confirms stability to the molecule. The C=C bond lengthens on hydrogenation from 1.34A to 1.55A. This means that van der Waals interactions such as the A13 eclipsing of the hydrogens can no longer occur, so the molecule loses stability. Torsional strain in the molecule also increases. On using MMFF94, a larger energies are obtained: 74.7 and 77.9kcal/mol for 1 and 2 respectively. These are much more similar in energy than from the MM2 calculation.
Interestingly, the taxol intermediate is unusual in that it is an "anti-Bredt" compound. An IUPAC definition of Bredt's rule is that: "a double bond cannot be placed with one terminus at the bridgehead of a bridged ring system unless the rings are large enough to accommodate the double bond without excessive strain". http://goldbook.iupac.org/B00732.html
These anti-Bredt compounds have been known about for a long time, and details of the synthesis of the first one can be found in J. Chem. Soc. 1979.[3]
Modelling Using Semi-empirical Molecular Orbital Theory
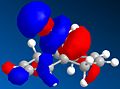

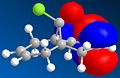
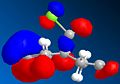
Since the previously used molecular mechanics approach cannot be used to investigate secondary (electronic) orbital interactions, another approach is now explored.
Regioselective Addition of Dichlorocarbene
MM2 calculations were used to minimise energy as before, giving a value of 17.9 kcal/mol. Next, orbitals were calculated with the MOPAC/pm6 method to give the following:
The method does distinguish between the two C=C, with the larger orbital in the HOMO being on the alkene bond nearest to the Cl. The orbitals are however unexpectedly assymetric with respect to the plane of symmetry of the molecule. On retrying the calculation, different orbitals were obtained (the HOMO is shown above). This is due to a defect in the MOPAC program code. A better method to use would be DFT.
Next a B3LYP/6-31G(d,p)Gaussian geometry optimisation and investigation of the frequencies was done to investigate the effect of the Cl on the double bond.

IR spectra were calculated for the original molecule, and a form where the double bond furthest from Cl was hydrogenated. The peak at 654 cm^-1 is contributed to by a wagging motion of the alkene hydrogens, and is therefore half the size in the spectrum for the hydrogenated molecule, and there is half the number of double bonds compared to the original molecule. The main C-Cl frequencies and C=C frequencies were identified using GaussView.
| vibration frequencies /cm^-1 | C-Cl | C=C |
|---|---|---|
| hydrogenated molecule | 782 | 1757 |
| original molecule | 773 | 1741 and 1761 |

This implies that the frequency for the double bond nearest to the Cl is higher, and therefore the bond is stronger than the other double bond. This suggests stabilisation due to the orbitals of the Cl, and supports the previous idea that the two alkene bonds are not identical. The C-Cl bond is weaker in the original molecule, which suggests that it is affected by both of the double bonds - even the one further away from it.
.
Structure based mini project using DFT-based MO methods
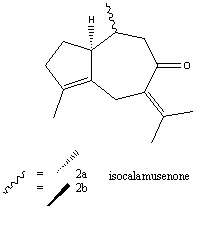
Introduction
Many naturally occurring bicycle[5.3.0]decane sesquiterpenes can be obtained from sweet flag oil (and other plant, liverwort and marine sources). In 1979,two sequiterpene ketones, and the similar tropone were isolated from sweet flag oil, and the major component, calamusenone, was characterised[4]. It is known to have both antinociceptive (reduces sensitivity to pain) and antimicrobial properties. The structure of the minor component, isocalamusenone, was partially identified; however the stereochemistry of the methyl group at C6 was not identified conclusively. Recently, Srikrishna et al. published a paper on “Enantiospecific first total synthesis and confirmation of the relative and absolute stereostructure of isocalamusenone”, having been investigating syntheses of natural products from (R)-limonene[5].
Methods
Methods used originally in the first characterisation were UV, IR, MS, 1H and 13C NMR, double resonance experiments, and europium reagent induced NMR. 1H and 13C NMR, and specific rotation were used by Srikrishna et al., as well as HRMS. These techniques led them to conclude that 2a is an epimer of the natural product, and 2b is an enantiomer (the optical rotation of 2b was found to be the same magnitude as that of the natural product, but the opposite sign). This mini-product aims to use computational techniques to confirm the stereochemistry of molecules 2a and 2b.
Geometry optimisation
This was done using the MM2 method in ChemBio3D, then sending this structure to be optimised by DFT methods. The following structure was found for 2b:
2b |
NMR
Next, 13CNMR were run on the optimised structures, and these were compared with the literature.
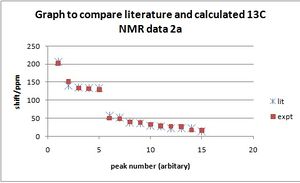
13CNMR(CDCl3) literature 2a: δ 206.5 (C, CO), 140.4 (C), 135.1 (C), 133.6 (C), 133.5 (C), 57.9 (CH, C-7), 51.3 (CH2, C-2), 38.2 (CH, C6), 36.5 (CH2, C5), 28.7 (CH2), 28.4 (CH2), 22.8 (CH3), 22.2 (CH3), 22.0 (CH3), 14.1 (CH3, C6–CH3);
experiment: δ 193.9(C4), 151.8(C13), 136.6(C1), 130.3(C10), 128.5(C3), 52.5(C7), 47.5(C5), 38.4(C6), 38.0(C9), 32.7(C2), 29.9(C8), 27.5(C14), 25.7(C12), 20.8(C15), 15.5(C11)
literature 2b:δ 210.0 (C, CO), 135.8 (C), 135.7 (C), 134.0 (C), 132.8 (C), 53.7 (CH, C-7), 47.5 (CH2, C-2), 37.1 (CH2, C-5), 31.0 (CH, C-6), 27.5 (CH2), 23.4 (CH2), 22.0 (CH3), 20.6 (CH3), 16.8 (CH3), 14.1 (CH3);
experiment: δ 201.8(C4), 151.8 (C13), 133.4 (C1), 131.8(C10), 128.7(C3), 50.4(C7),48.5(C5),39.7(C9), 38.0(C6), 32.9(C2), 28.4(C8), 27.6(C14), 26.2(C12), 17.1(C15), 15.8(C11),
It was noted that the only difference between 2a and 2b in the order of the size of the shift was for C9 and C6, with C6 being more shifted in 2b. This appears to follow the same pattern as the literature in that their 8th and 9th reported shifts are swapped over.
Next, an attempt was made to assign the unnamed carbons is the literature to the numbering used for the computational experiment. This was done by looking at the carbon type i.e. number of hydrogens attached to it. This was originally done by Shrikrishna et al. using DEPT-135. The following conclusions were made:
1. the four C type carbons were 1,3,4 and 10
2. the CH carbons were labelled as 6 and 7
3. two CH2 carbons were labelled as 2 and 5, and the other two were 8 and 9
4. one CH3 was labelled as 15, and the others were 11 12 and 14
5. C13 was not included.
Therefore, the carbons could be assigned as (in reverse order of shift magnitude) 4, 1, 10, 3 and 7. Thus far, the same order was found in all four data sets. The last five numbered carbons were similar in all sets: 8, 14, 12, and 11 and 15, with 15 having the smallest shift in the literature, and 11 in the calculation.
The middle section of shifts deserves a closer look, as this is where 2a differs from 2b.
| 2a lit | 51.3(C2 ) | 38.2(C6) | 36.5(C5) | 28.7(C9) |
|---|---|---|---|---|
| 2a expt | 47.5(C5) | 38.4(C9) | 38.0(C6) | 32.7(C2) |
| 2b lit | 47.5(C2) | 37.1(C5) | 31.0(C6) | 27.5(C9) |
| 2b expt | 48.5(C5) | 39.7(C6) | 38.0(C9) | 32.9(C2) |
C2, 5 and 9 are all CH2 type carbons, so their assignment may possibly be incorrect. The defining factor in comparing the literature 2a and 2b, and the calculated 2a and 2b appears to be the relative position of the magnitude of shift for C6. A smaller shift suggests product 2b in both cases. In this way, the literature suggorts the calculated NMR data. The results of the computation can be found in the following locations for 2a and 2b respectively: http://hdl.handle.net/10042/to-5663 http://hdl.handle.net/10042/to-5659.
3J H-H couplings were investigated using Janocchio, and inputted the optimised geometry model. Unfortunately, the values reported were different to those in the literature, for example, for 2a, the couplings for the hydrogen on C6 were caluculated as 10.2, 9.0 and 1.4 Hz, whereas literature values were 12.8, 8.4 and 4.8Hz. In 2b, no double doublet of doublets was given in the literature, however it was calculated by the program. This could be becasue the optimised geometry that was calculated is not exactly the true geometry, and, as the Janocchio instructions warn, "the couplings are very sensitive to the conformation".
IR

Next, IR spectra were run using the same programs as for the NMR. These were run as neat solutions in the literature experiment.
Literature 2a: mmax/cm^-1 2954, 2923, 2871, 1680 (C=O), 1619, 1456,1439, 1374, 1295, 1271, 1218, 1181, 1128, 1022 Computed 2a: {3170.9, 3115.8, 3065.6, 3025.5, 3007.0}, 1757.6(C=O), 1738.7 (small peak swallowed by C=O, but notable as it is the C=C peak for the alkene in the 5-membered ring), 1646.5 (C=C in 7-membered ring), {1308.9, 1227.0, 1204.0, 1178.9}. These were the main peaks - other smaller ones can be seen on the spectra. {}s denote peaks found in groups/close together.
Literature 2b: mmax/cm^-1 2955, 2927, 2876, 2842, 1678 (C=O), 1607, 1454, 1377, 1299, 1258, 1209, 1144, 1024;
Computed 2b: {3167.9, 3122.4-3105.9, 3068.7-3010.9}, 1760.2 (C=O), 1740.4(small C=C peak from 5-membered ring, as for 2a), 1648.3(C=C), {1314.8, 1225.3, 1173.0}
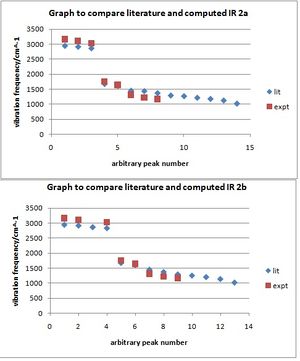
The computed values are silimar to the literature, however it would have been useful to know the value of intensity that was picked below which any "noise" was ignored. The lower intensity peaks at the smaller wavenumber end of the spectrum were not tabulated since they were so small, however the literature states more peaks. (The arbitrary scale on the graph should also be noted so that the comparitive purpose of the graph is not misleading).
Optical Rotation
Optical rotation was a key piece of information for determining the structure of natural isocalamusenone, and those synthesised in the literature. It was on this basis that 2a was found to be an epimer ( a diastereomer different at only one stereocentre), with [α]D 28 =-6.7 (c 0.7, CHCl3), and 2b found to be an enantiomer, with [α]D 28 = +176.2 (c 0.5, CHCl3). Since 2b had the opposite rotation to the natural product, it's configuration could be found.
These calculations were repeated computationally, however after running for nearly 2 days, the calculations were still not completed. Unfortunately these may have been mistakenly sent to the overnight scan and may have timed out.
Conclusion
The 13CNMR data suggests that the structures found in the literature were indeed the correct ones. The IR spectra suggests that the optimised conformations were very similar to the true ones, however discrepancies in frequencies suggest that they were not perfect. This is supported by the fact that the computed 3J H-H coupling constants did not match the literature. The deciding factor would have been the optical rotation, however unfortunately the results have not yet been computed.
More research into the properties of naturally occurring isocalamusenone with regards to antibiotic and other medicinal applications will hopefully be reported now that a synthesis from the easily accessible (R)-limonene has been published.
References and citations
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- ↑ The first anti-Bredt synthesis DOI:10.1039/C39790000082
- ↑ M Rohr, P Naegeli, J Daly, Phytochem., 1979, 18, 279.
- ↑ A Shrikrishna, V Pardeshi, K Mahesh, Tet. Asym., 2010, 21, 2512-2516. DOI:10.1016/j.tetasy.2010.09.008
