Rep:Mod:jc8717MM2
Molecular Modelling
NH3

Molecule: NH3 (Calculation Method: RB3LYP & Basis Set: 6-31G(d,p) )
Final Energy: -56.55776873 a.u.
RMS Gradient: 0.00000485 a.u.
Point Group: C3V
N-H Bond Length: 1.01798
H-N-H Bond Angle: 105.741 °
Charges: -1.125 on N atom and 0.375 on the three H atoms. Since N is more electronegative[1] than H, this result is to be expected.
Vibrational Analysis:
Expected Vibration modes: (3*4) - 6 = 6
There are two pairs of degenerate modes (2,3),(5,6).
3 modes are bend and 3 modes are stretch.
Which mode is highly symmetric? 4, symmetric stretch mode.
One mode is known as the "umbrella" mode, which one is this? 1, bending.
2 bands are expected to be seen in the IR spectrum of NH3; as vibrational modes 4,5 and 6 do not have a big enough change in dipole moment and vibrational modes 2 and 3 are degenerate.
Item Table from Log File: File:JC8717 NH3 OPTF POP.LOG
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986282D-10
Ammonia |
N2
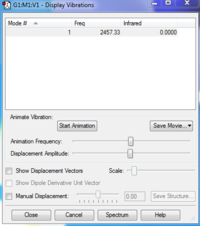
Molecule: N2 (Calculation Method: RB3LYP & Basis Set: 6-31G(d,p) )
Final Energy: -109.52412868 a.u.
RMS Gradient: 0.00000060 a.u.
Point Group: D*H
N-N Bond Length: 1.09200
Charges: 0 on both atoms, as expected for a homoatomic diatomic.
Vibrational Analysis: Being a linear diatomic molecule, it has one vibration type, a bond stretch. Since it is as well homo-atomic, there is no change in dipole moment and it does not give an IR signal.
Item Table from Log File: File:JC8717 N2 OPT.LOG
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401081D-13
Nitrogen Gas |
H2
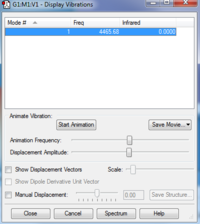
Molecule: H2 (Calculation Method: RB3LYP & Basis Set: 6-31G(d,p) )
Final Energy: -1.15928020 a.u.
RMS Gradient: 0.09719500 a.u.
Point Group: D*H
N-N Bond Length: 0.60000
Charges: 0 on both atoms, as expected for a homoatomic diatomic.
Vibrational Analysis: Being a linear diatomic molecule, it has one vibration type, a bond stretch. Since it is as well homo-atomic, there is change in no dipole moment and it does not give an IR signal.
Item Table from Log File:File:JC8717 H2 OPT.LOG
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
Hydrogen Gas |
Haber reaction
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.15928020 a.u.
3*E(H2)= -3.4778406 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.11356818 a.u(-298.17325659 kJ/mol)
The energy released by the formation of on mole of NH3 is 149.09 kJ/mol. This is an exothermic reaction, meaning that the products are more stable than the reactants.
Project Molecule
O2
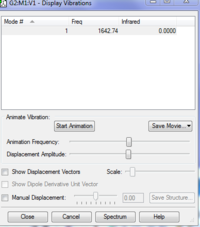
Molecule: O2(Calculation Method: RB3LYP & Basis Set: 6-31G(d,p) )
Final Energy:-150.25250603 a.u.
RMS Gradient: 0.05905207 a.u.
Point Group: D*H
O-O Bond Length: 1.21602
Charges: 0.000 on each O atom, as expected for a homoatomic diatomic.
Vibrational Analysis: Being a linear diatomic molecule, it has one vibration type, a bond stretch. Since it is as well homo-atomic, there is no change in dipole moment and it does not give an IR signal.
Item Table from Log File:File:JC8717 O2 OPT.LOG
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000130 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000113 0.001200 YES Predicted change in Energy=-1.033738D-08
Oxygen Gas |
Molecular Orbital Analysis
This MO is deepest in Energy. It is a non-bonding Molecular Orbital and has no effect on bond strength or bond order.

This MO is a sigma bonding orbital formed by the interactions between the 2s atomic orbitals of the O atoms.
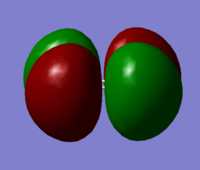
This MO is the HOMO, and it is a pi* anti-bonding orbital. This MO is formed by the destructive interaction between p atomic orbitals.

This MO is the LUMO, and it is also a pi* anti-bonding orbital. This MO is formed by the destructive interactions between p atomic orbitals, perpendicular to the previous MO.

This MO is a sigma* anti-bonding orbital, formed from the "head-on" destructive interactions of p atomic orbitals; this orbital has no electrons in it in the ground state configuration of O2.
CF4

Molecule: NH3 (Calculation Method: RB3LYP & Basis Set: 6-31G(d,p) )
Final Energy: -437.47454835 a.u.
RMS Gradient: 0.01113436 a.u.
Point Group: TD
C-F Bond Length: 1.32939
H-N-H Bond Angle: 109.471 °
Charges: 1.407 on C atom and -0.352 on the four F atoms. Since F is more electronegative[2] than C, this result is to be expected, as F will "pull" on the central carbon electrons and leave the central carbon with a positive charge.
Vibrational Analysis:
Expected Vibration modes: (3*5) - 6 = 9
Modes 1 and 2; 3,4 and 5; and 7,8 and 9 are degenerate to each other.
5 modes are bend and 4 modes are stretch.
Which mode is highly symmetric? 6 is a highly symmetric stretch .
2 bands are expected to be seen in the IR spectrum of CF4; as vibrational modes 1,2 and 6 do not have a change in dipole moment and vibrational modes 3, 4 and 4 are degenerate and modes 7, 8 and 9 are also degenerate.
Item Table from Log File: File:JC8717 CF4 OPT.LOG
Item Value Threshold Converged? Maximum Force 0.000078 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.000133 0.001800 YES RMS Displacement 0.000071 0.001200 YES Predicted change in Energy=-2.081640D-08
CF4 |
