Rep:Mod:jb4109(2)
Module 2
Optimising a Molecule of BH3
A molecule of BH3 was optimised. The gradient is the slope of the potential energy-distance graph, and a gradient less than 0.001 a.u. (Table 1) showed that the optimisation was complete. The "real" text-based .log file output was also checked to ascertain that the forces and displacements had converged.
| Optimised B-H Bond Distance | 1.19 Å |
| Optimised H-B-H Bond Angle | 120.0o |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Final Energy | -26.5 a.u. |
| Gradient | 4.51 x 10-5 a.u. |
| Dipole Moment | 0.00 D |
| Point Group | D3h |
| Calculation Time | 6.0 s |
Figure 1: Infrared (IR) spectrum of BH3

There are fewer than six peaks in the spectrum (Figure 1) even though there are six vibrations (Table 2), because the vibration at 2591.65 cm-1 has zero intensity, the two vibrations at 1204.86 cm-1 are degenerate, and the two vibrations at 2730.07 cm-1 are also degenerate. Hence, only three peaks are shown in the spectrum.
Using .chk file for the optimised BH3 molecule, a Full Natural Bond Orbital (NBO) analysis was performed ("pop=full"). [DOI:10042/to-13089 ] In the Formatted Checkpoint File, the occupied Molecular Orbitals (MOs) and Lowest Unoccupied Molecular Orbital (LUMO) were visualised (Figure 2). The .log file was then analysed for the NBO, and the charges are +0.3 and -0.1 for the B and H atoms respectively.
Figure 2: MO diagram of BH3
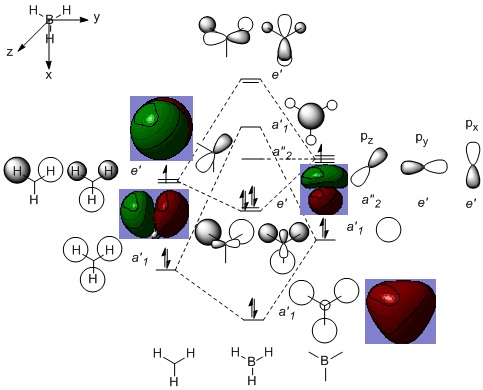
There are no significant differences between the real and Linear Combination of Atomic Orbtials (LCAO) MOs. The qualitative MO theory is useful in predicting the shapes, polarities and general relative energy levels of the MOs, but does not accurately reflect the specific energy levels and exact energy differences between the MOs. Gaussian computed BH3 with eight valence electrons, instead of six, so when the MOs were generated, there were four occupied MOs instead of three. The lowest occupied MO generated by Gaussian was ignored, because it was a lone pair of electrons occupying a boron sAO.
Using Pseudo-potentials and Larger Basis Sets for TlBr3
A molecule of TlBr3 was optimised with restricted D3h symmetry and a high tolerance value of 0.0001 (Table 3).
| Optimised Tl-Br Bond Distance | 2.69 Å, lit.[1] 2.56 Å |
| Optimised Br-Tl-Br Bond Angle | 120.0o |
| File Type | .log |
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Final Energy | -91.2 a.u. |
| Gradient | 8.80 x 10-7 a.u. |
| Dipole Moment | 0.00 D |
| Point Group | D3h |
| Calculation Time | 31.0 s |
The same method and basis set must be used for both calculations for energies to be compared, because the total energies of molecules are easily affected by these parameters. A frequency analysis has to be carried out to confirm that the optimised structure is a minima. The "low frequencies" for TlBr3 are: -3, -0.003, -0.0004, 0.002, 4, 46, 52 cm-1. The lowest "real" normal mode has a frequency of 46 cm-1 and 4 cm-1 in the infrared. Sometimes gaussview does not draw the bonds where expected, because the interatomic distance is greater than what the program has determined beforehand. A chemical bond is the electrostatic force of attraction force of one atom with another within a molecule. A covalent bond is the sharing of electron pairs between atoms. An ionic bond is Coulombic attraction between a cation and anion.
Isomers of Mo(CO)4L2
The ground state structures of cis- and trans-Mo(CO)4(PCl3)2 were optimised using the B3LYP method and LANL2MB basis set with loose convergence criteria ("opt=loose"). The torsional angles of the PCl3 ligands were changed in order to get to the lowest energy minima. The isomers were then optimised using the B3LYP method and LANL2DZ basis set with increased electronic convergence ("int=ultrafine scf=conver=9"). [Cis DOI:10042/to-12904 ; trans DOI:10042/to-12905 ]
The IR frequencies were calculated with the B3LYP method, LANL2DZ basis set and increased electronic convergence. [Cis DOI:10042/to-12939 ; trans DOI:10042/to-12938 ] Bond lengths: Mo-P 2.51 Å (cis), 2.44 Å (trans), lit. 2.42 Å; Mo-C 2.06 Å (both cis and trans), lit. 2.04 Å.[2] In the cis isomer, the Mo-P bonds are longer and weaker due to steric repulsion between the two PCl3 ligands at close proximity. Relative energies: -0.237(5080) kJ/mol (cis), -0.237(5076) kJ/mol (trans). The trans isomer is slightly more stable. This is because it has both PCl3 ligands far apart from each other as much as possible, so steric strain is minimised. The energy difference between these isomers is small (4 x 10-7 kJ/mol). We can use the ligand L = PH3, which has a smaller steric bulk, to try and stabilise the cis isomer. None of the vibrations have a very low or negative frequency (Figure 3).
Figure 3: Displacement vectors of low-frequency vibrations of cis- (top) and trans-Mo(CO)4(PCl3)2 (bottom)


The low frequency vibrations and rotations correspond to the molecule breathing, torsional and hindered rotational modes at room temperature. The IR spectral assignments are shown in Table 4 below. The experimental values were taken from 2nd year lab (Identification of Stereochemical Isomers of [Mo(CO)4L2] by Infrared Spectroscopy).
| Cis (cal.) | Cis (exp.) | Trans (cal.) | Trans (exp.) | |
|---|---|---|---|---|
| ν/cm-1 | 1945, 1949, 1958, 2024 | 1865, 1892, 1925, 2025 | 1951, 1951, 1978, 2031 | 1892, 2013, 2028 |
The cis isomer has vibrations of a slightly lower frequency than the trans isomer. Two of the vibrations of the trans isomer are degenerate, therefore the number of bands predicted from symmetry is correctly reflected in the experimental IR spectra.
Mini Project: Explore Bonding in Main Group Halides
The aim of this mini project is to:
- Determine the stability of the isomers of Al2Br2Cl4
- Investigate the effect of the Br atoms on the vibrational motion of the molecule
- Visualise the MOs of Al2Br2Cl4
Isomers of Al2Br2Cl4: I [AlCl2(µ-Br)2AlCl2, jmol file, DOI:10042/to-12989 ], II [AlBr2(µ-Cl)2AlCl2, jmol file, DOI:10042/to-12990 ], III [trans-AlBrCl(µ-Cl)2AlClBr, jmol file, DOI:10042/to-12991 ], were optimised with the B3LYP method and 6-31G(d) basis set. A frequency analysis was then performed to confirm that all three isomers have a minimum, i.e. all positive vibrational frequencies.
Relative energies: -2.84(49994) kJ/mol (I), -2.84(49976) kJ/mol (II), -2.84(49975) kJ/mol (III). The differences in energies is really miniscule. The lower the energy, the more stable the molecule is. Therefore, isomer I is most stable, followed by isomer II, and then followed by isomer III. From the similarity in energies between isomers II and III, it can be seen that stability has something to do with the bridging halogens. Isomer I is most stable, because the bridging Br atoms have a more diffuse electron cloud, and so can form stronger dative bonds to the Al atoms.
Figure 4: Isomer geometries

The data for terminal and bridging bond lengths agree with each other across the three isomers (Figure 4). It can be seen that bridging Al-X bonds are longer than terminal Al-X, because 3c-2e bonds are weaker than 2c-2e bonds.
| I | II | III | Lit.[3],[4] | |
|---|---|---|---|---|
| Terminal Al-Cl | 471, 602 | 534, 609 | 585 | 484, 625 |
| Terminal Al-Br | - | 413, 503 | 412 | 373, 500 |
| Bridging Al-Cl | - | 417 | 430 | 420 |
| Bridging Al-Br | 255, 366 | - | - | 198, 341 |
The vibrational frequency is inversely proportional to the square root of the reduced mass. Because Br (red atom) has a bigger atomic size than Cl (green atom), so the Al-Br vibrations has a smaller frequency than the Al-Cl vibrations (Table 5). This can also be seen from the smaller displacement vectors that the Al-Br vibrations have compared to the Al-Cl vibrations.
Figure 5: HOMO-6 (top left) to LUMO+1 (bottom right) of isomer I [DOI:10042/to-13019 ]

Figure 6: HOMO-6 (top left) to LUMO+1 (bottom right) of isomer II [DOI:10042/to-13020 ]
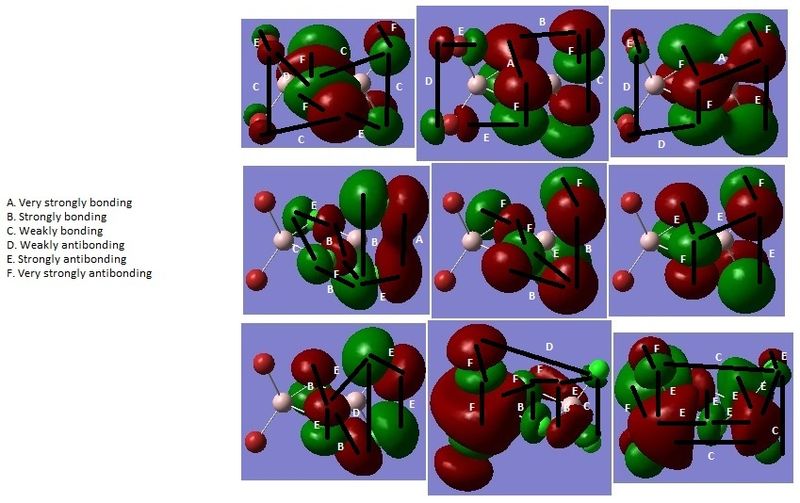
Figure 7: HOMO-6 (top left) to LUMO+1 (bottom right) of isomer III [DOI:10042/to-13021 ]
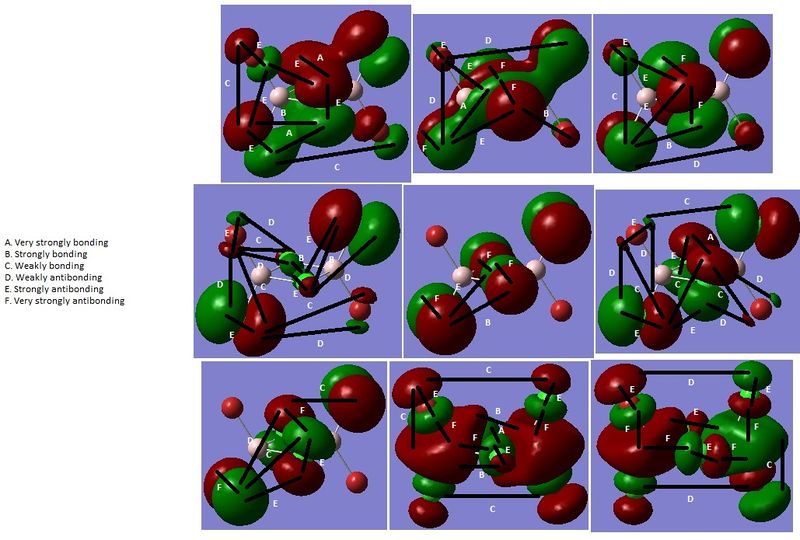
The LUMOs are more diffuse, and have more nodes, than the HOMOs (Figures 5-7).
Figure 8: Atomic charges of isomers

An NBO analysis was carried out, and the NBO charges are shown in Figure 8 above. A bridging halogen atom has a smaller negative charge than a terminal halogen atom, because in a bridging halogen atom, the two electrons are spread out over three atomic centres. Cl is more electronegative than Br, so the Cl atom has a greater negative charge than the Br atom. Consequently, an Al atom connected to four Cl atoms has the greatest positive charge, i.e. Al2 of isomer II (+1.4).
In conclusion:
- AlCl2(µ-Br)2AlCl2 is the most stable isomer, because of a larger electron cloud of the bridging Br, so stronger dative bonding to Al.
- ν(Al-Br) is smaller than ν(Al-Cl), because Br is a bigger atom.
- The MOs of Al2Br2Cl4 isomers were mapped and analysed.
References
- ↑ J. Glaser and G. Johansson, Acta Chemica Scandinavica, 36A, 125 (1982) [DOI:10.3891/acta.chem.scand.36a-0125 ]
- ↑ N.R. Champness, A.M. Hopkins and G. Reid, Acta Crystallographica Section C, 54, 797 (1996) [DOI:10.1107/S010827019501479X ]
- ↑ T. Onishi and T. Shimanouchi, Spectrochimica Acta, 20, 325 (1964) [DOI:10.1016/0371-1951(64)80030-8 ]
- ↑ D.M. Adams and R.G. Churchill, J. Chem. Soc. A, 2141 (1968) [DOI:10.1039/J19680002141 ]






