Rep:Mod:isi17
Inorganic Computational Lab - Iman Safia Ilyas
BH3 Molecule
Basis Set: 6-31G(d,p)
Method: B3LYP
Summary Table for BH3 Molecule
B-H Bond Length =
H-B-H Bond Angle = °
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000034 0.001800 YES RMS Displacement 0.000017 0.001200 YES
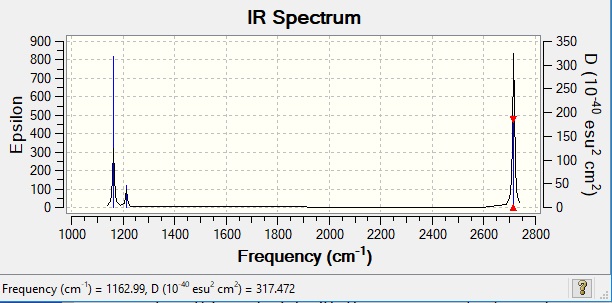
Low frequencies --- -2.2126 -1.0751 -0.0055 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
BH3 Molecule |
IR Analysis of BH3
| Mode | Vibrations (cm-1) | IR Active? | Intensity (Arbitrary Units) | Symmetry | Bend or Stretch |
|---|---|---|---|---|---|
| 1 | 1163 | Active | 93 | A2 | Out of Plane Bend |
| 2 | 1213 | Active | 14 | E' | Bend |
| 3 | 1213 | Active | 14 | E' | Bend |
| 4 | 2582 | Inactive | 0 | A1' | Symmetric Stretch |
| 5 | 2715 | Active | 126 | E' | Asymmetric Stretch |
| 6 | 2715 | Active | 126 | E' | Asymmetric Stretch |
There are less than 6 peaks in the spectrum although there are 6 modes of vibration. This is because not all modes are IR active. To be IR active there must be a change in dipole moment. Mode 4 is a symmetric stretch therefore is not IR active and is therefore not visible on the spectrum. The vibrations of modes 2 and 3 have frequencies very close to each other and therefore overlap showing one prominent peak. The same thing occurs for modes 5 and 6. The third peak is for Mode 1.
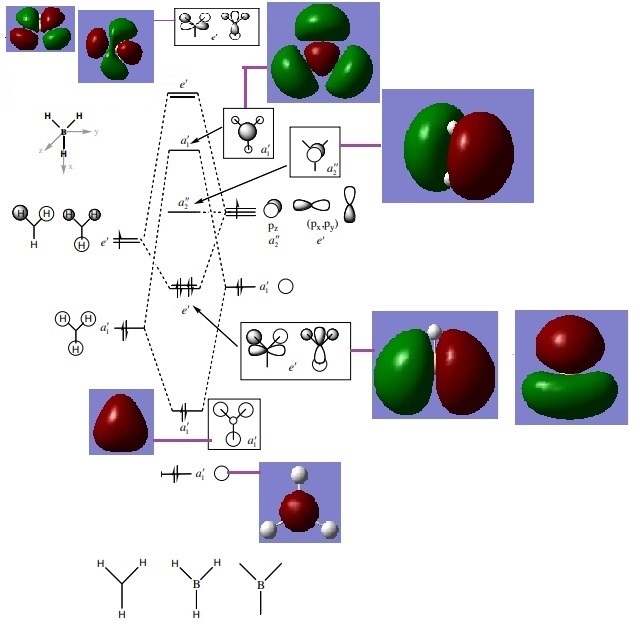
MO Analysis of BH3
(MO Diagram for BH3, Lecture 4 Tutorial Problem Model Answers, P. Hunt, Accessed 08/05/19 [1])
There are differences between the real MOs and the MOs predicted by LCAO, but they are not significant. Both have areas of high electron density and no electron density. Areas with no electron density represent nodes. By seeing the comparison between the real MOs and those predicted by LCAO, it can be said that MO theory is very accurate and useful. the differences are mainly the shape of the orbitals themselves. These are more distorted in the real MOs computed by gaussian.
Ng611 (talk) 20:50, 20 May 2019 (BST) Describe the differences you see!
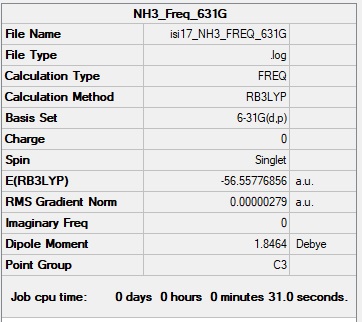
NH3 Molecule
Basis Set: 6-31G(d,p)
Method: B3LYP
Summary Table for NH3
N-H Bond Length =
H-N-H Bond Angle = °
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000006 0.001200 YES
Low frequencies --- -11.6527 -11.6490 -0.0048 0.0332 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
NH3 Molecule |
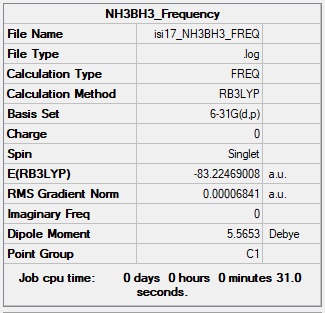
NH3BH3 Molecule
Basis Set: 6-31G(d,p)
Method: B3LYP
Summary Table for NH3BH3
B-H Bond Length =
N-H Bond Length =
B-N Bond Length =
H-B-H Bond Angle = °
H-N-H Bond Angle = °
H-B-N Bond Angle = °
H-N-B Bond Angle = °
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000338 0.001200 YES
Low frequencies --- -0.0010 -0.0006 0.0005 19.1023 23.7761 42.9934 Low frequencies --- 266.5969 632.3818 639.5198
NH3BH3 Molecule |
Energy Calculations
Association Energy:
Converting from au to kJmol-1 :
We can see that the N-B bond is relatively weak, especially if we compare this to a C-C bond with bond energy of 346 kJmol-1. The B-N bond has an energy less than half the bond energy of C-C.
Ng611 (talk) 20:51, 20 May 2019 (BST) Provide a reference for your literature bond value.
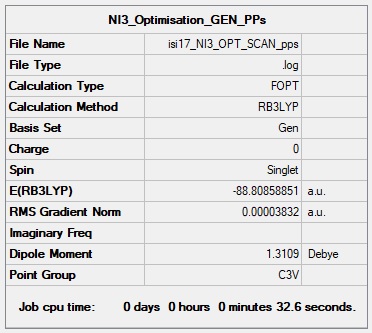
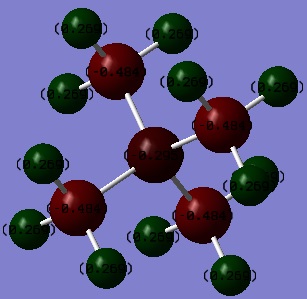
NI3 Molecule
Method: B3LYP
Basis Set: 6-31G(d,p) for Nitrogen, LanL2DZ (Pseudo Potential) for Iodine
My calculation took too long to complete on the SCAN server so I have included the Optimised LOG file and Summary Table from this file instead. Sorry for any inconvenience.
Summary Table of NI3
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000492 0.001800 YES RMS Displacement 0.000333 0.001200 YES
File:Isi17 NI3 OPT SCAN pps.log
Optimised N-I distance:
NI3 Molecule |
Ng611 (talk) 20:52, 20 May 2019 (BST) Your bond length is out by .004 A. Otherwise, good calculation.
Project: Ionic Liquids
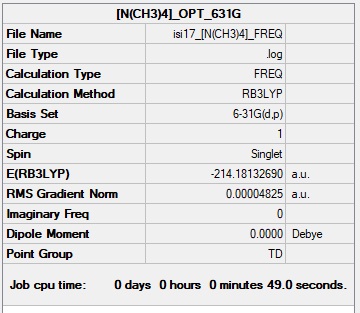
[N(CH3)4]+ Molecule
Basis Set: 6-31G(d,p)
Method: B3LYP
Summary Table of [N(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000072 0.000450 YES RMS Force 0.000029 0.000300 YES Maximum Displacement 0.000178 0.001800 YES RMS Displacement 0.000079 0.001200 YES
File:ISI17 -N(CH3)4- FREQ 01333599.LOG
Low frequencies --- -0.0011 -0.0007 -0.0007 35.5217 35.5217 35.5217 Low frequencies --- 217.0556 316.2155 316.2155
[N(CH3)4]+ Molecule |
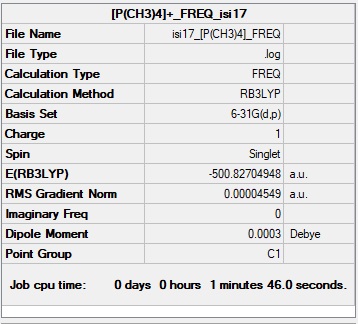
[P(CH3)4]+ Molecule
Basis Set: 6-31G(d,p)
Method: B3LYP
Summary Table of [P(CH3)4]+
Item Value Threshold Converged? Maximum Force 0.000095 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.001541 0.001800 YES RMS Displacement 0.000494 0.001200 YES
File:ISI17 -P(CH3)4- FREQ 01333599.LOG
Low frequencies --- -0.0003 0.0018 0.0026 13.7497 22.4514 35.0103 Low frequencies --- 162.9535 193.8816 195.5982
[P(CH3)4]+ Molecule |
Comparison of Charge Distributions of [N(CH3)4]+ and [P(CH3)4]+
Image 1: Shows Charge Distribution of [N(CH3)4]+
Image 2: Shows Charge Distribution of [P(CH3)4]+
The Charges of the Individual Atoms in [N(CH3)4]+ are:
Charge on the Nitrogen atom = -0.295
Charge on each Carbon atom = -0.484
Charge on each Hydrogen atom = +0.269
The sum of the partial charges is: +0.997
The Charges of the Individual Atoms in [P(CH3)4]+ are:
Charge on the Phosphorous atom = +1.667
Charge on each Carbon atom = -1.060
Charge on each Hydrogen atom = +0.298
The sum of the partial charges is: + 1.003
The colour range used was from -1.667 to +1.667, where green represents a positive charge and the red represents a negative charge. This was due to the fact that Phosphorous held the highest charge of +1.667 out of any atom in either of the systems. This range was kept constant for both cations, so that direct comparisons of the charges on each atom could be made. It can be seen that the Phosphorous atom holds a much more positive charge than the nitrogen in their respective cations. This can be explained as Nitrogen (3.04) is a much more electronegative element than Phosphorous (2.19) on the Pauling scale. this means that Nitrogen is more likely to pull the electron density towards itself in the N-C bond thus having a more negative charge on the atom. The charge is also more negative on the carbon atoms in [P(CH3)4]+ than in [N(CH3)4]+ as the carbons (2.55) in [P(CH3)4]+ are the most electronegative atoms in the cation therefore hold more of the negative charge, whereas in [N(CH3)4]+ they are bonded to an even more electronegative atom, nitrogen, which withdraws some electron density. [P(CH3)4]+ has a higher overall positive charge (+1.003) than [N(CH3)4]+ (+0.997) but they both round to +1.00 if we take them at 2 decimal places.
Ng611 (talk) 20:55, 20 May 2019 (BST) Good! A discussion about symmetry and perhaps some discussion about the hydrogen partial charge.
Validity of Traditional Description of [N(CH3)4]+
If we look at the traditional description, the formal charge is said to be localised solely on the nitrogen atom, however, it can be seen that it actually holds part of a negative charge. The positive charge is spread around the outside of the molecule as it is delocalised around the hydrogen atoms. The Hydrogen atoms are where the actual positive charge is located for this molecule.
Ng611 (talk) 20:58, 20 May 2019 (BST) Why is this the case?
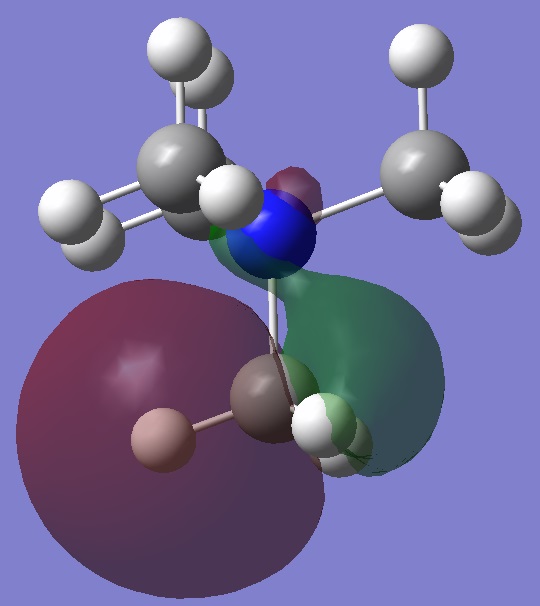
Occupied Valence MOs Showing Bonding and Anti-bonding Character
Valence MO 6
This is a bonding MO and has 2 nodes, one between the nitrogen and carbon and one between the carbon above and the hydrogen atoms attached to it. We can see sigma bonding here as 2 lobes are interacting in phase alone the bond.
Valence MO 15
This MO has 1 node which can be seen as the area with no electron density between the large green and red lobes. This MO is an anti bonding orbital.
Valence MO 21
This MO has 1 node, which can be seen as the area with no electron density between the large red and green lobes, which are out of phase with each other. This MO is an anti bonding orbital.
Ng611 (talk) 20:56, 20 May 2019 (BST) You're missing LCAO analyses for MOs 16/21 (which look fairly similar to one and other). A detailed MO analysis for all orbitals (highlighting key interactions) would have improved this answer significantly.