Rep:Mod:ir208
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
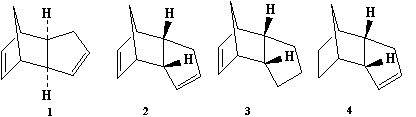
Cyclopentadiene dimerisation
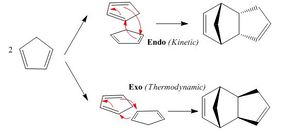
Cyclopentadiene molecules slowly dimerise at room temperature via [4+2] cycloaddition namely the Diels-Aler reaction. The resultant dimer conformation is dependent of the relative orientation of the cyclopendadiene acting as dienophile and the one acting as diene. The exo approach corresponds to the substituent on the dienophile being directed away from the diene whist the endo approach to the the substituent on the dienophile being directed towards the diene.
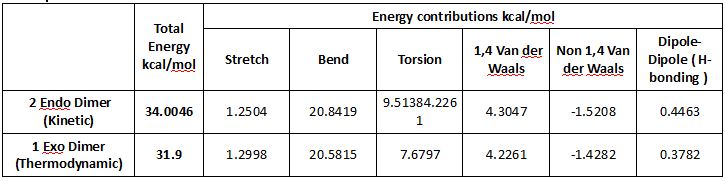
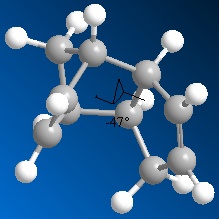
The thermodynamic stability of the the exo dimer(1) compared to the endo dimer (2) may be rationalised considering the dihedral angle between the bridging over the 6-membered ring and the 5-membered ring. In the exo case this angle is 177 o whilst in the endo case it is 47 o . Repulsive steric interactions whith the cyclopentene rings being closer together raise the energy of the endo dimer over compared to the exo one. In the exo case, a nearly antiperiplanar arrangement of the ring carbons over two connecting carbons (ideally 180o), reduces torsional strain and prevents steric clashes between the rings. Torsional strain is indeed computed to be greater in the endo case.
The Diels-Alder reaction of two cyclopentadienes is nonetheless mostly stereoselective towards the endo dimer. Computational modeling, shows that the exo dimer is thermodynamically more stable by a few kcal/mol than the endo dimer. The relative stability of the transition states directs the stereoselectivity and the Diels-Alder reaction is therefore under kinetic control. Several hypotheses have been made to explain the endo stereoselectivity.
Alder suggested that a “plane to plane” orientation of reactants maximising the accumulation of double bonds the promotes a complex formation, which may rationalise the endo selectivity of the reaction. [1] Woodward and Hoffmann proposed that the HOMO-LUMO interactions in the endo transition state were more favourable than in the exo transition state due to orbital symmetry. The black dashed line s represent the main orbital overlap responsible for bonding. The endo selectivity is assigned to the secondary overlap represented by the red lines. Such orbital overlap is nonetheless not proven to be as important is herein depicted.[2]
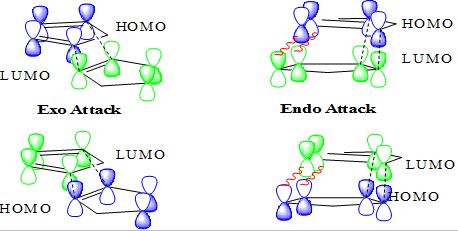
A further hypothesis explaning the endo selectivity of the reaction invokes the transition state resulting from a asynchronous reaction mechanism. The zwitterionic resonance structure of the transition state brings charges closer together in the endo case than in the exo case and thereby favours the endo dimerisation.[3]
Hydrogenation of the dimer
The hydrogenation of the cyclopentadiene dimer proceeds via a two step mechanism. Only one of the of the double bonds is hydrogenated initially forming either dihydro derivative 3 or 4. The reactivity of the two double bonds in the cyclopentadiene dimer are different leading to the selectivity in the first hydrogenation step and mainly related to torsional strain.
In the norbodene moity, the sp2 hybridised carbon both exhibit bond angle of about 108°, whereas in the cyclopentene ring sp2 hybridised carbon exhibit bond angles of about 114°. In the cyclopentene, ring the sp2 hybridised carbon thus have bond angles much closer to the optimum value of 120°, implying a more stable double bond. The norbodene double bond is more prone to hydrogenation than the cyclopentene one explaining the selectivity towards dihydroderivative 4.

Initial hydrogenation of the norborene double bond generates the more stable product, namely structure 4. The cyclopentene double bond is then hydrogenated in a second step. The reaction is thermodynamically controlled by the stability of the generated product. The hydrogenation of the norborene double bonds relieves considerable bending strain and accounts for the greater stability of structure 4. The bending term is the prevalent contribution to the difference in energy between structure 3 and 4.

Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
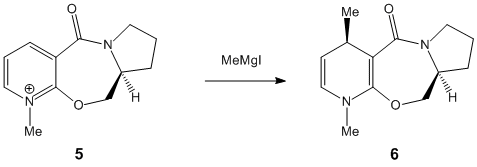
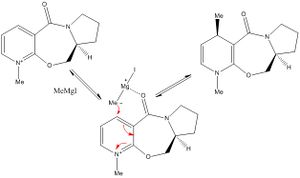
The nucleophilic addition of MeMgI to the optically active derivative of prolinol (5) results in the alkylation of the pyridine ring in the 4-position, with the absolute stereochemistry shown in 6. The pyridinium ring of 7 is derivatised by reaction with aniline in the 4-position to form 8. The latter may acts as NHPhenyl group transfer agent to electrophiles such as carbonyls in a reverse of the reaction by which it was formed. In both cases, the relative stability of the reactant conformers, more specifically the orientation of the carbonyl group with respect to to the pyridinium ring plane, provides stereocontrol to the addition reaction. A MM2 forcefield is used for the optimisation geometries starting from different reactant conformations, a few of which are depicted below (for each case). Optimisation of reactant geometries provides mechanistic insight to the reactions. The Griggnard reagent MeMgI is however omitted from any energy optimisation as the Magnesium atom is not recognised by the MM2 forcefield used.
Nucleophilic addition to the chiral propinol derivative
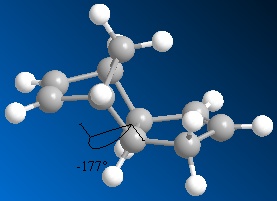
The seven membered lactam ring in the derivative of prolinol is in fact quite rigid, and the optimisation of different stereomodels yielded mostly very similar structures. Two conformational minima respecting the absolute stereochemistry are nonetheless to be distinguished regarding the orientation of the carbonyl group with respect to the aromatic ring. The amide carbonyl group is either in the plane of the ring or, in the case of the most stable conformer, above that plane anti to the hydrogen atom at the chiral center. The dihedral angles between the ring and the carbonyl ranged between 18o and 25o for the carbonyl above the pyrindinium ring plane and the total energy between 41 and 44 kcal/molo . For the co planar conformer, bond angle were around 0o energies around 50 kcal/mol.
Coplanar orientation of the lactam carbonyl
Upwards orientation of the lactam carbonyl
Upon application of the variational principle, the lowest energy conformer is considered as the prevalent reactant. Carbonyl group orientation then provides region and stereocontrol to the nucleophilic addition of the methyl group by coordinating +MgI. Such chelation forces the nucleophilic addition to occur at the 4-position above the plane of the pyridinium ring. The C=O group being anti to the proton at the chiral centre in the propinol derivative 5 suggests that the stereochemistry at this centre controls the conformation of the seven membered carbonyl ring and thus the regio and stereo selectivity of the reaction[4].
Nucleophilic addition to the chiral quinolinium compound 7
The chiral quinolinium compound 7 reacts via nucleophilic addition with aniline at the 4-position to form 8, a so called “amide transferring agent”[5]. The reaction exhibits diastereofacial selectivity as a result of the steric control induced by the lactam carbonyl group. Optimatisation of the quinolinium conformers is here used to predict such selectivity. The flexibility of the lactam ring is particularly poor due to its adjoining phenyl groups. Most investigated conformers were high in energy and showed considerable strain and are thus highly improbable. The conformational minima exhibits the lactam carbonyl below the plane of the pyridinium ring with a dihedral angle of approximately 20o.
Downwards orientation of the lactam carbonyl
Co-planar orientation of the lactam carbonyl


As they both contain lone pairs of electrons, there would be lone pair lone pair repulsions, which would direct the incoming nitrogen group away from the oxygen atom; hence onto the other side of the ring to the oxygen. You can also see that if the nitrogen group were to attack the same side as the oxygen there would be a great deal of steric hindrance which would also prevent the attack at this point. The aniline therefore reacts on the opposite side of the lactam carbonyl giving rise to the afore mentioned diastereofacial selectivity. Lone pair -lope pair repulsions between the carbonyl oxygen and the aniline nitrogen may explain why the amine reacts on the opposite face with respect to the carbonyl. Dipolar coupling between the the methyl group of the lactam and the aniline proton has been reported [6].and further reinforces the proposed direction of attack. The C3 (pyridinium ring) C=O chiral axis is the main structural feature controlling the selectivity of the reaction.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
An important intermediate of the total synthesis of taxol, an anticancer drug examplary of the power of synthetic and protecting group strategy investigated here. In the synthesis reported by Paquette [7] this key intermediate 9 or 10 is initially synthesised with the carbonyl group orientated either up or down, and isomerises to the alternative carbonyl isomer upon standing. This examplifies atropisomerism, namely the distinction between 2 configurations arising from the restricted rotation about a single bond caused by high steric demands of the substituents. The stereochemistry of carbonyl addition is induced by the relative stability of these isomers.
Atropisomerism of the intermediates


Different conformers of these isomers are investigated empirically for their stability using MM2 force field optimisation. For both isomers 9 and 10, the conformation of the cyclohexane ring is key. Further manual alterations to the positions of the atoms and re-minimisation of the energy were insignificant. In most cases, the different starting conformations of the cyclohexane ring, be it twist boat, boat , or chair convert to to the chair conformer upon optimisation. Overall, only three significant structures respecting of the absolute stereochemistry of the intermediates atropisomers were found. Intermediate 10, that is to say with the carbonyl group pointing down, was modelled for the cyclohexane ring in its chair and twist boat conformation, the chair conformation being significantly lower in energy. Intermediate 9, with the carbonyl group pointing up could only be modelled with the cyclohexane ring as a twist boat in order to respect the prescribed stereochemistry. Atropisomer 10 is overall more stable than atropisomer 9. The results of MM2 and MMFF94 forcefield optimisations are presented in the table below. MM2 and MMFF94 results are consistent regarding the relative stability of the atropisomers.

The higher energy atropisomer 9 it its twist-boat conformation is synthesised initially, and then isomerised on standing to the more stable atropisomer 10 in its chair conformation. The rigidity of the cyclo-transannular ring structure inhibits cyclohexane conformational interchange for each isomer, locking 9 into the higher energy twist-boat conformation and 10 into the chair conformation.
Hyperstability of the bridgehead alkene
The molecule here investigated exhibit relatively poor alkene reactivity. The bridgehead alkenes in 9 and 10 are hyperstable due to conformational ring stain induced by the 10 membered ring. At a bridgehead in a large rigid ring system, an sp2 carbon is more favourable than an sp3 one, such Hydrogenation of the bridgehead alkene to the parent hydrocarbon is therefore not favoured as it would induce more strain. Intermediates 9 and 10 are described as anti-Bredt molecules referring to the non adherence to Bredt's Rule. The latter is the empirical observation is based on Bredt's rule which states that a double bond may not be located at the bridgehead of a bridged ring system (unless the ring is large enough).[8]
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
MOPAC Molecular orbital theory optimisation
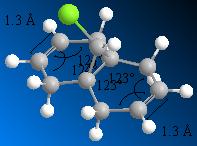

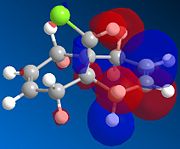
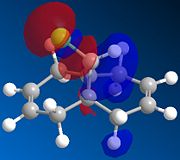


A molecular orbital investigation of the structure and π-facial regioselectivity of chloro-tetrahydro-methanonaphtalene, compound 12, is presented here. Orbital interactions, provide regio- and stereocontrol to the reaction. The naphtalene derivative 12 reacts with electrophilic reagents such as dichlorocarbene regiospecifically at the double bond endo to the chlorine substituent on the opposite face to the cyclopropyl ring. [9].Orbital interactions between the diene and the electrophile/nucleophile, provide regio- and stereocontrol to the reaction. Molecular orbitals are modelled using a semi-empirical molecular orbital theory method know as MOPAC/PM6. The latter method generates an approximate picture of valence molecular wavefunctions.
Structure of 12
Optimisation of the naphtalene derivative geometry using MOPAC allows to distinguish between the two double bonds. The presence of the chlorine group induces geometrical distortions of the two rings such that the two double bonds have inequivalent bond angles. The implied electrostatic π-facial asymmetry provides stereocontrol. Halter et al. Proposed that such asymmetry stabilises the antiperiplanar interaction between the C-Cl σ* orbital and the exo π-orbital, mainking the endo double bond more nucleophilic.
The molecular orbital approximations calculated demonstrate the above asymmetry and the two double bonds are clearly to be considered as distinct environment . The HOMO, HOMO-1, LUMO, LUMO +1, depicted below are significantly asymmetric with respect the central ring linking bond.
The HOMO concentrates most electron density around the double bond endo to the chlorine atom. The endo double bond is thus very electron rich and hence nucleophilic. The HOMO-1 nonetheless shows much electron density on the exo double bond orbital. The HOMO-1 is more stable and more delocalised than the HOMO due to its antiperiplanar overlap with the C-Cl σ* orbital (LUMO +2) and therefore less reactive. Halter et al. Quantified the latter stabilisation to be of about 0.08 eV (ref). This explains the endo selectivity of the reaction with electrophiles.
Gaussian Density-functional vibrational calculation
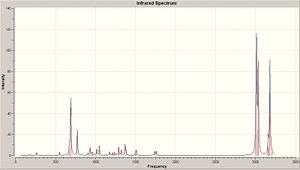

The previously optimised chloro-naphtalene derivative 12 and its parent dihydrogenation product are subjected quantum mechanical optimisation method, in order to calculate the vibrational frequencies of molecules. Indeed Gaussian Density-functional B3LYP/6-31G(d) method accounts for real life movements within a molecule. C=C and C-Cl vibrations are particularly significant.
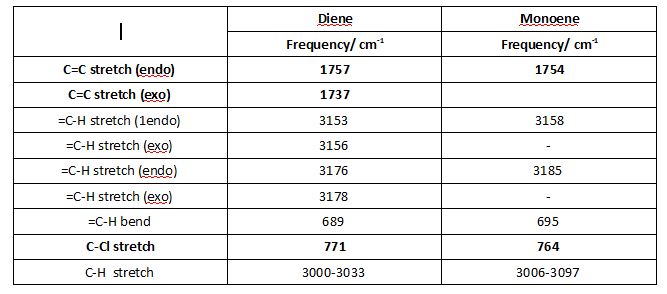
C-Cl stretch vibrations are found at 771 cm-1and 764 cm-1 for the diene and the monoene respectively. C=C bond vibrations yield are the most intense IR absorptions. In the diene, two C=C stretching frequencies are observed at 1757 cm-1 and 1737 cm-1 for the endo and exo double bonds respectively. Only one C=C stretching absorption corresponding to the endo double bond is observed for the monoene at is observed 1754 cm-1. Significantly, the exo double bond exhibits a lower stretching frequency than the endo one the diene. The aforly mentioned molecular orbital interactions rationalise this observation. The interaction between the exo π-orbital and the C-Cl σ* reduces electron density at the exo C=C π-orbital, reducing the exo bond order and thus the vibrational frequency. The reduced bond order of the exo double bond explains why the dihydrogenation product is left a double bond endo to the Cl. The monoene observed had the double bond anti to the C-Cl bond hydrogenated. Both molecules exhibit Cs symmetry, yet differ in their dipole moment, the latter being larger for the monoene than for the diene.
Structure based Mini project using DFT-based Molecular orbital methods
Assigning Regioisomers in "click chemistry" with reference to the Huisgen reaction.
Click chemistry is a chemical philosophy aiming at synthesizing substances quickly and reliably by joining small units together just as in nature. The concept was introduced by Nobel Laureate K. Barry Sharpless in the early 21st century upon realisation that the addition of a Cu(I) catalyst renders the 1,3-dipolar cycloaddition between an azide and an alkyne to give a 1,2,3-triazole extremely facile. Substituted alkynes and azides yield two possible regioisomers , namely the 1,4-Isomer A and the 1,5-isomer B. Under Cu(I) catalysis, the 1,4-isomer A prevails whilst Ru(II)-catalysed reactions give predominantly the 1,5-isomer B instead. [10].The Huigsen 1,3-dipolar cyclo addition exploits this concept very well and methods to discriminate between A and B are investigated here.

13C NMR is the most effective spectroscopic method available to differenciate regioisomers A and B. The Infrared Spectra of Regioisomers A and B would be similar since they exhibit the same functional groups. IR analysis is thus not a particularly useful discriminating technique in this case and has not been computed here.
1H NMR would probably not be the best means to differienciate A and B since most protons are aromatic. The latter having very similar resonances, overlap and complex multiplicity patters are to be expected. Further, the methylene protons would have very similar shift thus not offering an alternative identification means. In order to compute NMR caculations, the geometry of the regioisomers must be optimized initially using MM2 forcefield and MOPAC/PM6. Further optimisation was obtained from a Gaussian SCAN using a 6-31G(d,p) basis set, whose output may be fed back again into SCAN for an NMR calculation.
The jmol applets bellow show that the two regioisomers exhibit very different geometries. In 1,4-regioisomer A, the phenyl and triazole ring are nearly aligned in the same plane, whilst in 1,5-regioisomer B they are nearly perpendicular. This influences their spectroscopic properties.

The obtained C-NMR data clearly allows to distinguish regioisomers A and B on three fundamental points, namely the the chemical shifts of the unsubstituted triazole carbon, the phenyl substituted triazole carbon and the methelene carbon.
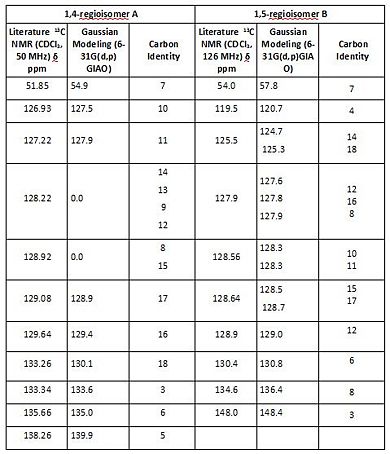
The unsubstituted triazole carbons shift is more deshielded in 1,4-regioisomer A than in 1,5-regioisomer B due to different adjacent nitrogen environment. In A C3 is found at 133.6ppm whilst in the 1,5 isomer (B) it is more shielded at 120.7 ppm. In 1,5 isomer B, C1 is adjacent to a Nitrogen whose lone pair is delocalised in the heteroaromatic aromatic ring and thus doesn’t deshield C1 as much as in 1,4 isomer A. In Regioisomer A, the unsubstituted triazole carbon (C3) is located next to a nitrogen whose lone pair is localised in the sp2 hybrid orbital rather than delocalised within the aromatic triazole ring. The lone pair deshields C3 to 133.6ppm. Presence of a resonance at about 120 ppm is therefore selectively representative of B.
The phenyl substituted triazole carbon differ in their chemical shift due to their respective alignment with the plane of the phenyl ring. In A it is found at at 139.9ppm whilst in the 1,5 isomer (B) it is more deshielded at 148.4.ppm.
The phenyl group is part of an extended planar π delocalised system which deshields the adjacent carbon. As can be seen from the aforly mentioned optimised structures, in A the carbon is coplanar with the phenyl ring whilst in B it is not. A is thus more deshielded due to a better alignment with the plane of the phenyl ring. The presence of a chemical shift at about 148 ppm, or indeed lack thereof, may be used to differenciate regioisomers A and B, its presence being selectively representative of B.
The NMR data presented in the original paper by Sharpless[12], includes resonances at 119ppm and 148 ppm from which it may be concluded that it corresponds to 1,5-regioisomer B.
The literature NMR data
[13] [14] correlates very well with the calculated data, but not perfectly. The differences observed relate probably to the ininitial structural optimisations, rather than subsquent NMR carculations. Computational predictions allow to distinguish every single carbon. Experimentally, this is not possible since peaks resulting from similar environment are often found to overlap. Computational modeling methods are extremelly powerfuls synthetic tools nonetheless may fail to describe the true complexity of the experimental analysis and NMR assignments.
References and citations
- ↑ K. Alder and G. Stein, Liebigs Ann. Chem., 1933, 504, 210-257.DOI:10.1002/jlac.19335040115
- ↑ The conservation of orbital symmetry.R. Hoffmann, R. B. Woodward Acc. Chem. Res. , 1968 , 1 , 17 - 22DOI:10.1021/ar50001a003
- ↑ Mechanistic aspect of Diels-Alder reactions : A Critical Survey.J. Sauer, R. Sustmann Angew. Chem., Int. Ed. Engl. , 1980 , 19 , 779 - 807DOI:10.1002/anie.198007492
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838.DOI:10.1021/jo00356a016
- ↑ AS. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.DOI:10.1016/j.tetasy.2004.11.004
- ↑ AS. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.DOI:10.1016/j.tetasy.2004.11.004
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319.DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 110.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ A. B. McEwen, P. v. R. Schleyer, J. Am. Chem. Soc.,1986 108 (14), 3951-3960 DOI:[https://doi.org/10.1021/ja00274a01 10.1021/ja00274a01 ]
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ J. Am. Chem. Soc. 2005, 127, 15998 DOI:10.1021/ja054114s
- ↑ V. N. Kizhnyaev, F. A. Pokatilov, N. A. Tsypina, G. V. Ratovskii, L. I. Vereshchagin, A. I. Smirnov, Synthesis of N-Vinyl-1,2,3-triazole Derivatives, Russian Journal of Organic Chemistry, 2002, 38, 7, 1056-1059DOI:10.1023/A:1020874201056
- ↑ S. Chuprakov, N. Chernyak, A. S. Dudnik, V. Gevorgyan, Org. Lett., 2007, 9, 2333-2336DOI:10.1021/ol070697u
- ↑ Bull. Korean Chem. Soc. 2010, Vol. 31, No. 7 1843 DOI:10.5012/bkcs.2010.31.7.1843
- ↑ S. Chuprakov, N. Chernyak, A. S. Dudnik, V. Gevorgyan, Org. Lett., 2007, 9, 2333-2336DOI:10.1021/ol070697u
