Rep:Mod:inorganic complab1
Introduction
The aim of this lab is to be able to understand the reason why certain basis sets are used in computational chemistry and what results they give. The energies, IR frequencies and MO’s of the molecule will be investigated to see if computational chemistry gives an output that is accurate in accordance to the theory. Also computational chemistry will be used research an inorganic molecule and conclusions will be drawn from the results obtained. To be investigates are the IR frequencies of BCl3, the MO's of BH3 and the cis and trans isomerism and hows this affects the stretching frequencies of a certain functional group in the octahedral complex [Mn(CO)4(PCl3)2]
Chemical Formula: https://www.ch.ic.ac.uk/wiki/index.php/Image:POP_GAB_BH3.LOG
BCl3 IR Analysis
The calculation method used is DFT, B3LYP and the basis set is LanL2MB which is a medium level basis set. This information was obtained from the summary file in GaussView. According to this summary the calculation took only 19 seconds. Even though this caculation was done very quickly, it does not mean that it ha failed or is inaccurate. The molecule in question is a very simple one and was already in its preferred trigonal planar structure before it was optimised. Also the point group symmetry used is corecct so the program does not have to make up for this and thus the caculation runs quickly.
The reason that the same method and basis set must be used for each calculation is that the frequency is the second derivative of the optimisation. Therefore the two things must be kept the same in order to get a correct result.
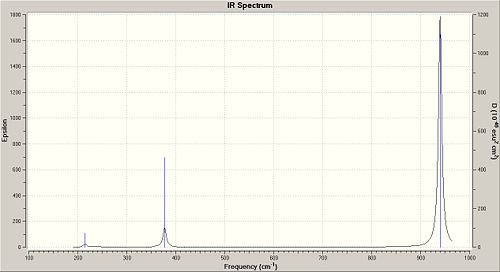
We need to carry out a frequency calculation in order to show that our molecule is a minimum. If all of the frequencies are positive in value then it is indeed at a minimum. However if there is one negative value then this implies that the structure we have is in fact a transition state and has not been fully optimised. All of the frequecies obtained for BCl3 were positive so we know that the moecule is at a minimum and all of the calculations have worked.
The optimised B-Cl bond length was 1.8Å, and the literature value for the bond length is 1.75Å. This is not exactly the same, but it is a good approximation as Gaussview is not entirely accurate so two significant figures will suffice for bond length values. Therefore the two bondlengs are very similar and are only out be 0.05Å.
BCl3 is a trigonal planar molecule with bond angles of 120° and a point group of D3h. Its bond angles were looked at in the optimised molecule and these too were 120°.
A bond is an interaction between atoms or molecules that allow for the formation of polyatomic compounds. It is the attraction between two opposing charges or dipoles (δ+ δ-). These opposite charges are attracted to each other via electromagnetic force, i.e the electrons and protons are attracted to each other. More simply a chemical bond is the attraction between atoms that result from a sharing of electrons.
The symmetry expected from the ground state structure is D3h and this is the point group that GaussView used to complete the calcultaions. This suggests that the output is accurate as it used the same point group that is expected from theory.
MO analysis of BH3
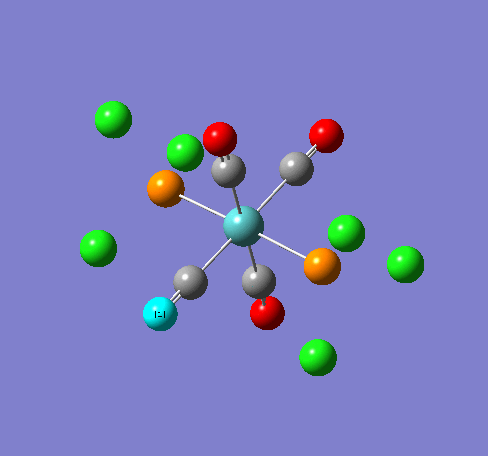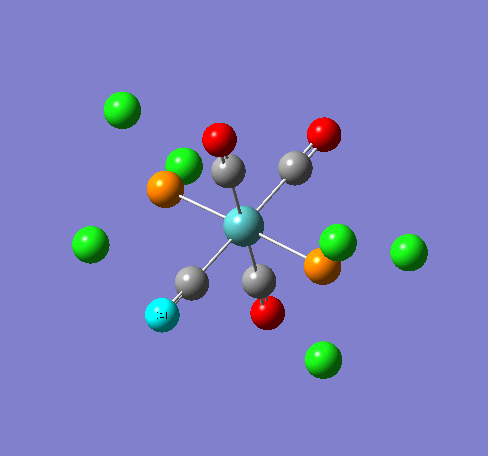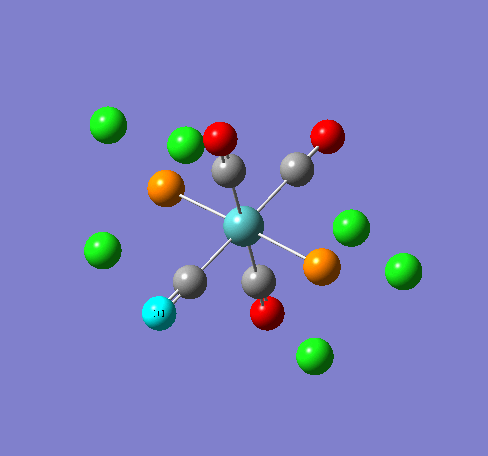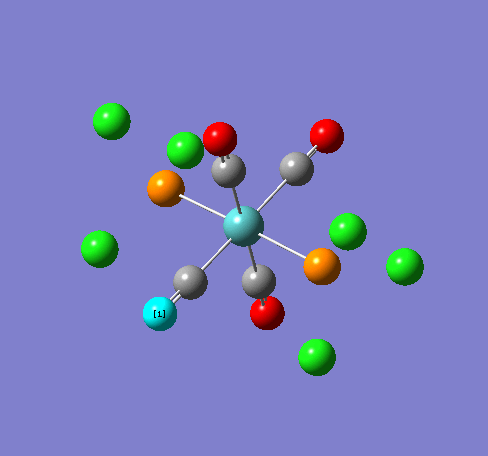
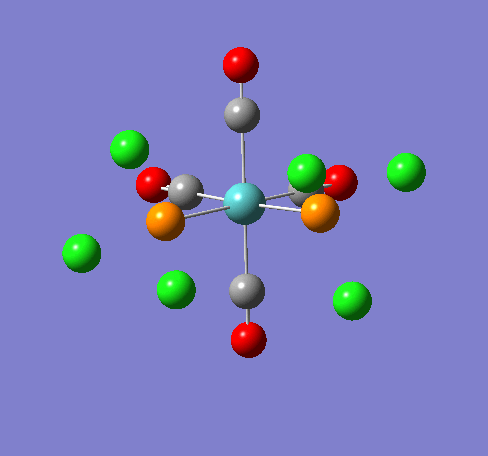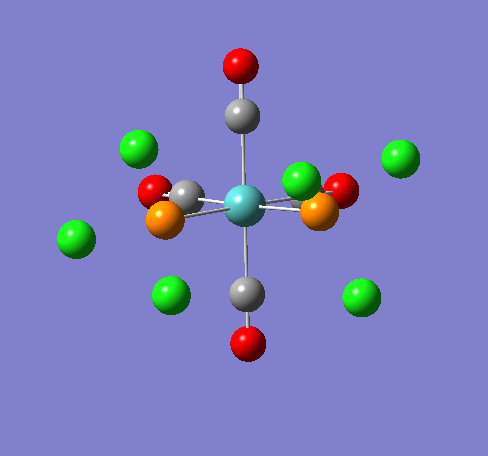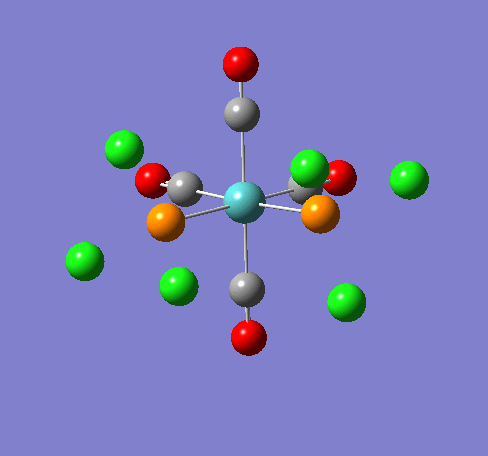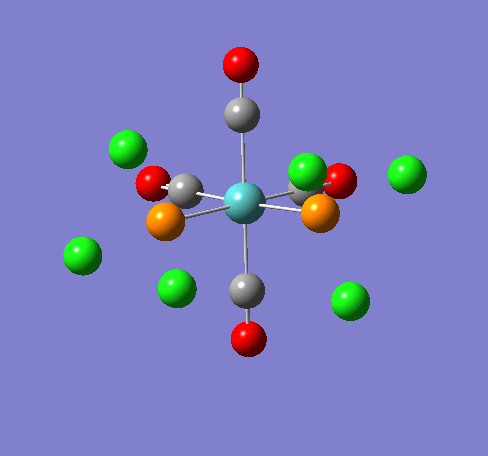
Isomers of Mn(CO)4(PCl3)2
Isomer Stability
According to the summary taken from the calculation in Gaussview the trans isomer is more stable. However there is only an energy difference between the cis and trans isomer of 4kJ/mol. The error within the program is ~5kJ/mol so this energy difference is not large enough to tell us whether or not one isomer is more stable than the other. With an energy difference as low as this it can be concluded that both the isomers are at the same energy, neither is significantly higher or lower in energy than the other.
One would expect the trans isomer to be more stable. The reason for this is because the C=O groups is electron withdrawing. In the trans structure all of these groups are situated in the same plane of the structure. They are all in the same environment and are symmetrical. Therefore the C=O will take electrons out of the d orbital’s on the central Mn thus weakening the overall structure.
In the cis isomer the C=O groups are not symmetrical, i.e. there will be a PCl3 group trans to one of the C=O ligands. As P is electron donating the structure is stabilised as the phosphorous will donate electrons into the d orbitals on the metal as the C=O is withdrawing them. Therefore this isomer is theoretically more stable.
There could also e some favourable orbital interaction that stabilises one of the isomers over the other but that would be a whole different experiment and will have to be investigated at a different time.
The ligand PR3 can be changed so that the relative ordering of the cis and trans isomers will be altered. On way of doing so, so that the trans ligand is favoured is to make R very bulky, for example R=Ph or tButyl. The two ligand will want to be as far apart as possible to reduce the steric clash and so they will go trans to each other when they attach to the central metal.
Another factor that can be looked at to alter the relative ordering is the trans effect of the R group.
IR Vibrations
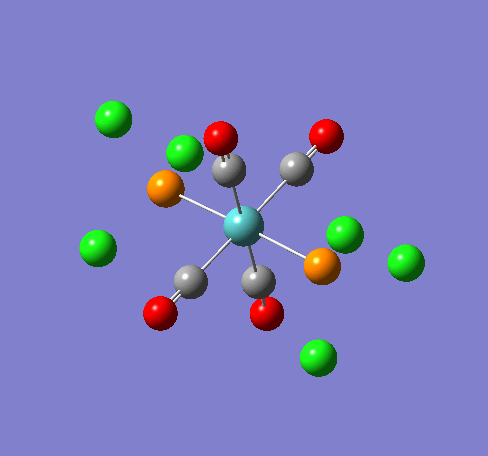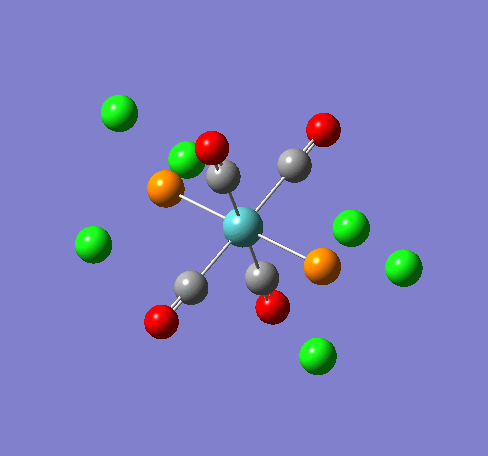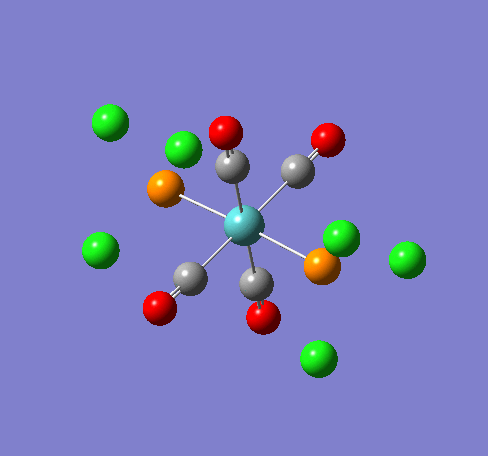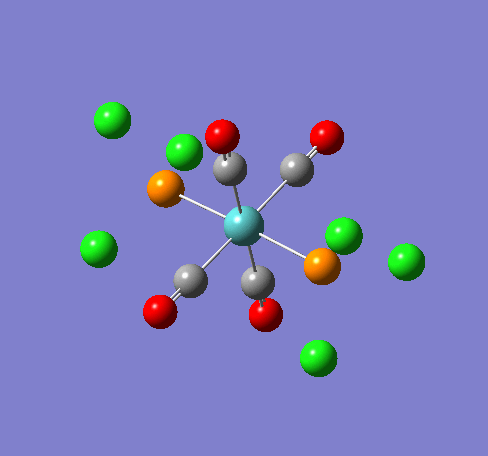
Some of the vibrations for the two isomers had very low frequencies at 10cm-1 <. The Trans isomers had two low frequencies at 4cm-1 and at 6cm-1, and the cis isomer had a low frequency of 10cm-1. These are shown in the table below.
| Trans 4cm-1 | Trans 6cm-1 | Cis 10cm-1 |
|---|---|---|
 |
 |
|