Rep:Mod:inorganic complab
Introduction
The aim of this lab is to be able to understand the reason why certain basis sets are used in computational chemistry and what results they give. The energies, IR frequencies and MO’s of the molecule will be investigated to see if computational chemistry gives an output that is accurate in accordance to the theory. Also computational chemistry will be used research an inorganic molecule and conclusions will be drawn from the results obtained. To be investigates are the IR frequencies of BCl3, the MO's of BH3 and the cis and trans isomerism and hows this affects the stretching frequencies of a certain functional group in the octahedral complex [Mn(CO)4(PCl3)2]
Chemical Formula: https://www.ch.ic.ac.uk/wiki/index.php/Image:POP_GAB_BH3.LOG
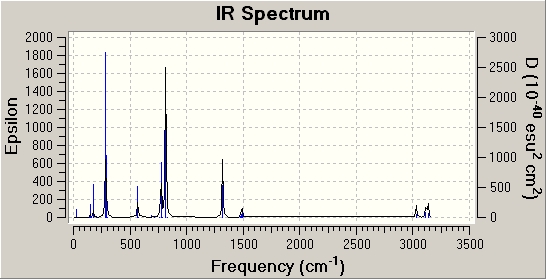
BCl3 IR Analysis
The calculation method used is DFT, B3LYP and the basis set is LanL2MB which is a medium level basis set. This information was obtained from the summary file in GaussView. According to this summary the calculation took only 19 seconds. Even though this caculation was done very quickly, it does not mean that it ha failed or is inaccurate. The molecule in question is a very simple one and was already in its preferred trigonal planar structure before it was optimised. Also the point group symmetry used is corecct so the program does not have to make up for this and thus the caculation runs quickly.
The reason that the same method and basis set must be used for each calculation is that the frequency is the second derivative of the optimisation. Therefore the two things must be kept the same in order to get a correct result.
We need to carry out a frequency calculation in order to show that our molecule is a minimum. If all of the frequencies are positive in value then it is indeed at a minimum. However if there is one negative value then this implies that the structure we have is in fact a transition state and has not been fully optimised. All of the frequecies obtained for BCl3 were positive so we know that the moecule is at a minimum and all of the calculations have worked.
The optimised B-Cl bond length was 1.8Å, and the literature value for the bond length is 1.75Å.[1]
This is not exactly the same, but it is a good approximation as Gaussview is not entirely accurate so two significant figures will suffice for bond length values. Therefore the two bondlengs are very similar and are only out be 0.05Å.
BCl3 is a trigonal planar molecule with bond angles of 120° and a point group of D3h. Its bond angles were looked at in the optimised molecule and these too were 120°.
A bond is an interaction between atoms or molecules that allow for the formation of polyatomic compounds. It is the attraction between two opposing charges or dipoles (δ+ δ-). These opposite charges are attracted to each other via electromagnetic force, i.e the electrons and protons are attracted to each other. More simply a chemical bond is the attraction between atoms that result from a sharing of electrons.
Therefore it does not matter if GaussView shows the bond drawn into the molecule or not. The bond drawn is just a way for us to visulaise the structure. As GaussVie will know that a sharing of electrons is taking place between the two atoms in question, then the calculations will be done with this taken into account. It therefore does not matter that there may not be a bond drawn in GaussView.
The symmetry expected from the ground state structure is D3h and this is the point group that GaussView used to complete the calcultaions. This suggests that the output is accurate as it used the same point group that is expected from theory. Gaussview allows you to set the symmetry and when it runs the optimisation the symmetry will not change. Gaussian will not break the symmetry that you set it to be.
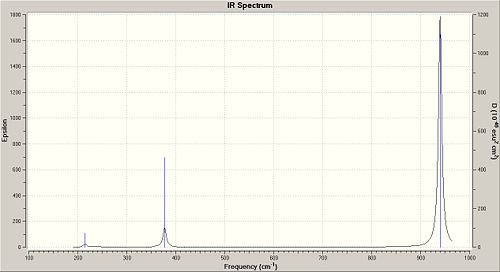
The reason that there are only three peaks in the spectrum is because two of the peaks are degenerate and one has zero intensity. The frequency at 2598.42cm-1 is at zero intestity because it is totally symmertric so there is no overall change of dipole moment so there is no peak in the IR spectrum. References
- ↑ 1. C. Spencer and W. N. Lipscomb, J. Chem. Phys. 28, 355 (1958)
MO analysis of BH3
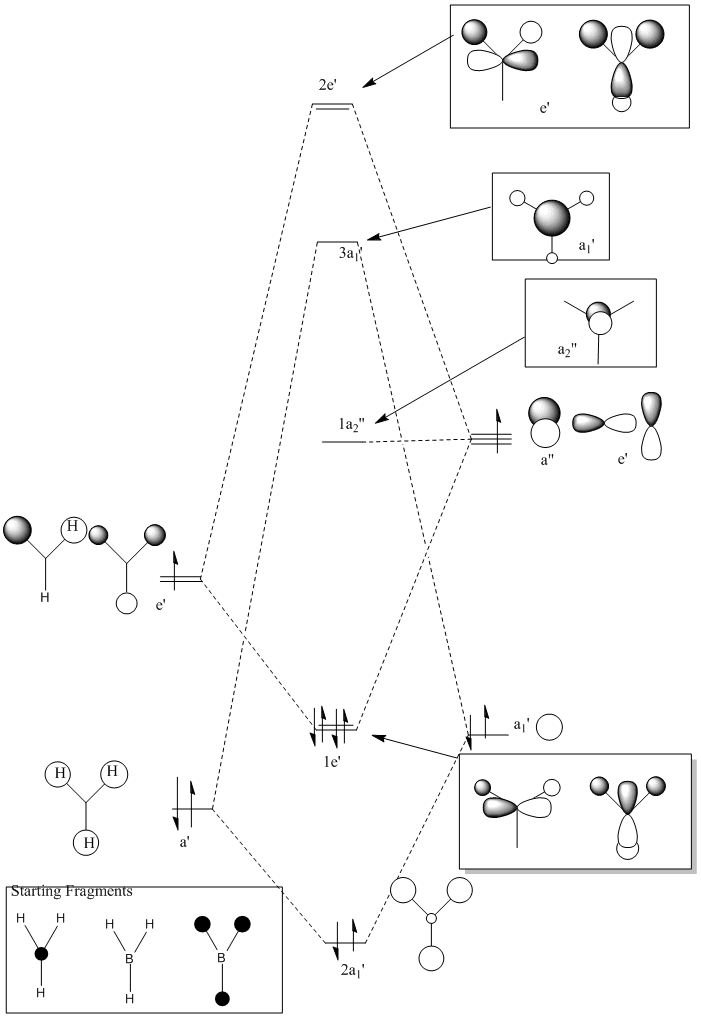
The MOs for BH3 were calculated and all calculations were run on Gauss View. The molecule was loosely optimised and then fully optimised. The checkpoint file was obtained and the MO were analysed from this.
The calculation type was SP, the calculation method was RB3LYP and the basis set used was 3-21G. The total energy of the molecule was -16605.2847kJ/mol (-26.4622 a.u).
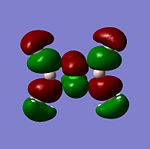
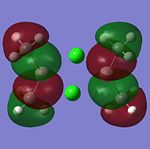
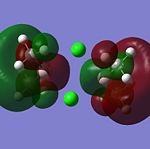
The MO’s were then compared against the LCAO’s and the results were mixed.There is another MO that sits below this diagram as it is at a much lower energy to the others, at -4223.14kJ/mol. The first MO on the diagram, at the lowest energy looked very similar to the all bonding a1' orbital. For the 1e' MO's, the different nodes can clearly be seen and so this gives a good approximation from GaussView that agrees with the LCAO's for this energy level.These two orbitals are degenerate, as can be seen from their E symmetry, but also from the fact they are at the same energy of -223.96kJ/mol. The MO of a2' symmetry is also well reprsented by GaussView and this orbital is at an energy of -46.81kJ/mol. It is from this orbital onwards that a diference in the orbitals appreance becomes apparant from the LCAO and the GaussView calculations. These orbitals are also at much higher energies compared to the other orbitals and are thus less stable. The energy for thee orbitals were 118.46kJ/mol The computed MO’s were well matched against the LCAO’s for the HOMO’s but for the LUMO’s they looked slightly different. The reason for this is that it will be hard to predict the shape of the LUMO’s because they are a concept before they are filled. The computational method makes the LUMO’s more diffuse. The computer does not know how to handle this. Therefore the HOMO’s are an accurate representation of the MOs but the LUMO’s are not.
The HOMOs are very accurate and if you look at the MO diagram you can see that the MO’s obtained from the computational calculation are very close to the LCAO’s. However the LUMOs are not as accurate.
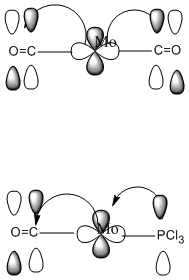
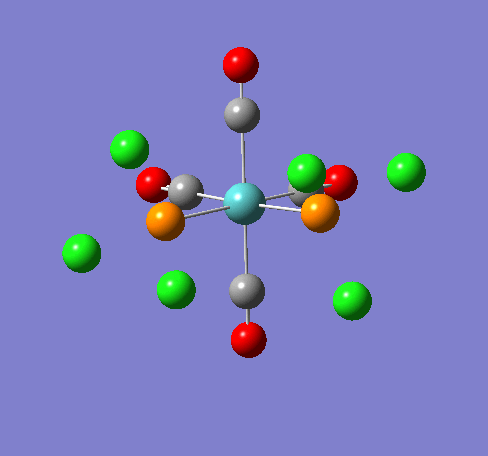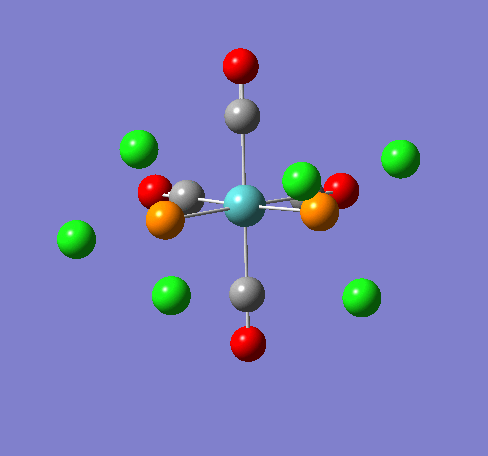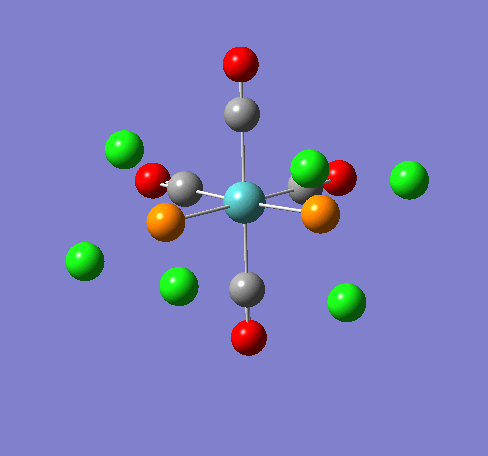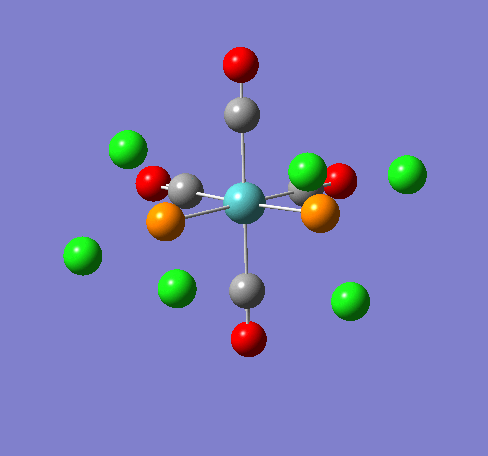
Isomers of Mo(CO)4(PCl3)2Isomer Stability[1] According to the summary taken from the calculation in Gaussview the trans isomer is more stable. However there is only an energy difference between the cis and trans isomer of 4kJ/mol. The error within the program is ~5kJ/mol so this energy difference is not large enough to tell us whether or not one isomer is more stable than the other. With an energy difference as low as this it can be concluded that both the isomers are at the same energy, neither is significantly higher or lower in energy than the other. One would expect the trans isomer to be less stable. The reason for this is because the C=O groups is electron withdrawing. In the trans structure all of these groups are situated in the same plane of the structure. They are all in the same environment and are symmetrical. Therefore the C=O pi antibonding orbital will take electrons out of the d orbital’s on the central Mn thus weakening the overall structure. In the cis isomer the C=O groups are not symmetrical, i.e. there will be a PCl3 group trans to one of the C=O ligands. As P is electron donating the structure is stabilised as the phosphorous will donate electrons into the d orbitals on the metal as the C=O is withdrawing them. Therefore this isomer is theoretically more stable. There could also e some favourable orbital interaction that stabilises one of the isomers over the other but that would be a whole different experiment and will have to be investigated at a different time. The ligand PR3 can be changed so that the relative ordering of the cis and trans isomers will be altered. On way of doing so, so that the trans ligand is favoured is to make R very bulky, for example R=Ph or tButyl. The two ligand will want to be as far apart as possible to reduce the steric clash and so they will go trans to each other when they attach to the central metal, as for these ligands to attactch to the metal in the cis formation is sterically unfavrorable and will make the complex much less stable. Another factor that can be looked at to alter the relative ordering is the trans effect of the R group. The bond lengths for the optimised cis and trans structures from GaussView are as follows:
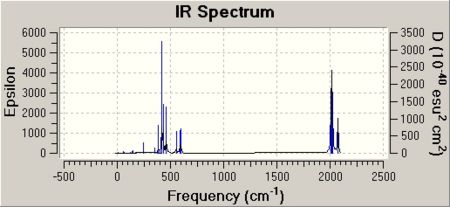
Some of the vibrations for the two isomers had very low frequencies at 10cm-1 <. The Trans isomers had two low frequencies at 4cm-1 and at 6cm-1, and the cis isomer had a low frequency of 10cm-1. These are shown in the table below, and illustrated with diagrams.
These vibrations are happeneing at room temperature. Having a frequency this low suggests that these rotations are accessable at room temperature. The PCl3 groups are freely rotating at room temeprature, and the diagram at oom temeprature shows this. The symmetry of the molecules predicts the amount of C=O stretches that will be seen in the IR spectrum. As all of the C=O in the trans molecule are in the same plane then this suggests that there is no difference between the C=O environments and so we would predict only one peak in the Ir spectrum.The point group of this structure from the literature is D4h and the expected number of peaks from this is 1. From the symmetry of the molecules you would expect the cis isomer to have multiple IR stretching frequencies for the C=O stretch as there are multiple C=O environments. These are the C=O trans to each other, the C=O cis to each other, the C=O trans to the PCl3 groups and the C=O cis to the PCl3 group.From the literature the point group of this molecule is C2v and fromthis symmetry label 4 peaks are to be expected.[2] The C=O vibrations for the two isomers are displayed below. the literature values quoted are taken from experimantal data from the year two labcourse. They are the results that I obtained for cis and trans Mo C=O IR frequencies. All frequencies throughout this wikipedia page are given in wavenumbers :
The results obtained from GaussView are actually very similar to the experimental data from the Year2 labcourse. From the spectra below in the cis spectrum three C=O peaks can be clearly seen and this agrees with the prediction. However 4 frequencies were obtained but one of these were at zero intensity (noted above) and so it will not show up on the spectrum. The trans spectrum shows two distinct peaks. Three were noted but there are only 4 wavenumbers in between them and so this can be counted as the same peak. Symmetry predicted only one peak, and only one was obtained in the experimental.
Mini Project-Study of the stability of Aluminium dimers vs their corresponding monomersThis project involves studying Aluminium halide dimers and comparing their energies to their corresponding monomers, and other dimers that have the same halide. The aim is to see if the dimer is more stable than two units of the monomer. Also to see how changing the R groups to a more bulky one affects the stability and also how changing the halide affects the stability. The molecules that are to be investigated are Al2Br2Me4, Al2Cl2Me4, Al2Cl2Ph4 and Al2Br2Ph4. All the corresponding monomers will also be looked at and their energies obtained. To do this we must first loosly optimise the structure using the basis set LanL2MB, then to fully optimise the structures using the more precise basis set LanL2DZ. The energies of the fully optimised structures will then be noted and compared so that a conclusion can be drawn. One of the structure needs to be fully analysed using Frequency and MO analysis. The molecule to be analysed is the dimer Al2Cl2Me4 Al2Cl2Me4 was loosely optimised and then was sent to scan so that the vibrational frequencies could be obtained, and the MO’s could be analysed.DOI:10042/to-2716 The vibrational spectrum of Al2Cl2Me4 was obtained, and according to the literature peaks should be seen at 300-600cm-1 for the Al-Cl [3] bonds and peaks should be seen at 665-760cm-1 for Al-C [4] bonds. When the spectrum was looked at closely this is exactly what was found. There were many other peaks present of course but these were the ones of note. These vibrations are shown below along with their relative intensities.
This shows that the correctly optimised structure has been obtained for the dimer and that GausView has correctly calculated the IR frequencies as in this case the frequencies fully match up with the literature values. There are no negative frequency so this shows that the structure reached a minimum. DOI:10042/to-2714
The HOMO shows antibonding characteristics, as all of the lobes are in different phases. Around the Cl atom a p-type orbital can be seen, the same as with the Al. The carbons seem to be showing s-type character.The bridging and terminal groups show antibonding characteristics. The HOMO-1 shows all of the lobes in opposite phases too. There are no orbitals around the Cl, but again there seems to be p-type orbitals around the Al.As mentioned theere are no orbitals on the Cl and this is quite unusual. One would expect all of the atoms in the system to be involved in the bonding. The HOMO-2 shows bonding characteristics between the H and Cl, P and S orbitals. Also on the pictured MO bonding characteristics can be seen around the C and Al atoms. The terminal groups show antibonding lobes and the bridging groups show bonding characteristics. Like before the LUMOs do not give enough significant information about the molecule, as it was found out for the analysis of BH3. As GaussView is not equipped to deal with unoccupied orbitals, then they are not an accurate representation of what the actual LUMOS of the molecule would look like.
The monomer of Al2Cl2Me4 will now be compared to the dimer. The aim of this is to decide if two units of the monomer are more stable than the dimer. This will show if the dimer is the preferred, lower energy structure. AlClMe2 has an energy of -96.84Hartrees, so two monomer units have an energy of -193.7 Hartrees. Comparing this to the dimmers energy of -193.7308 Hartrees. We can see that when we convert the energy difference into kJ/mol the dimer is at a lower energy with an energy difference of 107.65kJ/mol. This confirms that the dimer is at a lower energy. Next we can compare the AlBrMe2 monomerDOI:10042/to-2717 with the dimer. The monomer has an energy of -94.04624Hartrees, and so two repeat units have an energy of -188.1Hartrees. The dimer has an energy of -190.1455kJ/mol. This give an energy difference between the two structures of 5390.42kJ/mol. Again the dimer is much lower in energy and this is to be expected. The energy of dimerisation can now be compared between these two dimers. The energy of dimerisation for Al2Cl2Me4 is 107.65kJ/mol and for Al2Cl2Me4 it is 5390kJ/mol. The energy for dimerisation is much lower for the dimer containing the Al-Cl bond. The reason for this is the electronegativity difference between the Cl -and Br-. Cl- is much more electronegative than Br and so there will be a larger polarity difference between the Al and the Cl- and thus a stronger bond is formed. The orbital overlap is also a factor and this has been explained above. This first part of the study has shown that the dimer is at a lower energy that its corresponding monomer. Now this theory is to be tested by comparing the energies of Al2Cl2Ph4 and its monomer and also of Al2Br2Ph4 and its monomer. This is to see if changing the R group has any effect over the stability of the dimer and two repeat units of the monomer, and also to see what changes it makes to the energy change of dimerisation. Firstly Al2Cl2Ph4 was compared to AlClPh2. The monomer has an energy of -480.24859847Hartrees so two repeat units of the monomer has an energy -960.5Hartrees. The dimer has an energy of -960.527Hartrees. The energy difference(and hence the energy of dimerisation) is 18.7kJ/mol. Next Al2Br2Ph4DOI:10042/to-27019 was compared to AlBrPh2. The monomer has an energy of -472.819Hartrees, so two repeat units of this monomer have an energy of -945.6hartrees. The dimer has an energy of -956.9408Hartrees. Again the dimer has a lower energy and the energy difference in this case is 7092.67kJ/mol. The dimerisation energy of the two chlorine containing complexes will now be compared. The reason for this is to see if changing the R group has any effect over the preference for the monomer to dimerise or if it is more stable in the monomeric form. The energy difference between two monomer units and the dimer with R=Me was 107.65kJ/mol, but when R=Ph the energy difference was 18.7kJ/mol. The latter is a much lower energy and this suggests that the larger the R group the more stale the monomer is. If the R group was even more bulky than Ph then the monomer would be the preferred structure over the dimer.[5] The energy of dimerisation is so much higher for the bromine containing molecules than it is for the cholrine containing morecles. There is a much lower energy barrier to overcome for a chlorine containing monomer to dimerise than there is for a bromine containing monomer. the reasosn that this could be are explained below. One reason for this is that Chlorine and Aluminium are in the same row in the periodic table. This would mean that they are corectly alligned for a favourable bonding situation to occur. Bromine is in the row below aluminium and chlorine and so this is not as favourable an interation.  The energy of dimerisation is much larger for both Br containing molecules when R=Me and Ph. This agrees with the electronegativity of the molecule, and the charge distribution can be shown. For Cl, the Muliken charge was given as -0.446, but for Br it was -0.397. This explains why the energy of dimerisation is so high. The bromine is less electronegative and is at a higher energy so it is easier to react. This therefore weakens the structure and makes the Al-Br dimer less stable, according to the energy difference of dimerisation. The reason for this can be explained using the NBO analysis of the molecule.
As the Br is less electronegartive than the Cl s will not induce as much polarisation between it and the Al, which results in a weaker bond. As the Cl is higher in electronegativity a more polar (and more ionic) stronger bond is produced thus stabilising the dimer and this was shown in the experimetal results for the energies of dimerisation as this energy for the Cl containing molecule is much lower. So in conclusion this study has found that the molecules studied are more stable in the dimer form, as two repeat units of the monomers have a higher energy than the dimer. Also the energy of dimerisation was compared and it was found that this was lower for Al2Cl2R(Me or Ph)4 than Al2Br2R(Me or Ph)4. Therefore it can be predicted that if the halide was I-, then the energy of dimerisation would be much higher as you go down the rows in the periodic table then the overlap between Al and X becomes less favourable. This was shown for Cl and Br in the diagram. This study could be taken further and all of the energies of dimerisation could be investigated for aluminium dimers with the different halides as the bridging atom. Another way to take this study further is to change the metal. The argument has been made that the reason the energy of dimerisation for the dimer with Br as the bridging atom is so much higher than that for the dimer with Cl as the bridging atom is because it is in a different row to Al. This theory could be tested by finding out the energy of dimerisation of Ga2Br2Me4 and comparing it to the monomer. And also of In2I2Me4 to see if there is a trend in the energy of dimerisation for molecules in the same row on the periodic table. References
|