Rep:Mod:inorganic2124
Structural Analysis of BCl3
The structure of BCl3 was drawn out in Gaussview, shown in figure 1, and the following calculations were carried ou: the optimisation of geometry (finding the lowest energy of the molecule), the frequency calculation (to find the vibrational spectrum of the molecule, amnd finding the length of the B-Cl bond in the molecule.
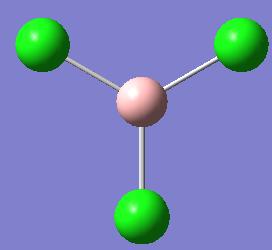
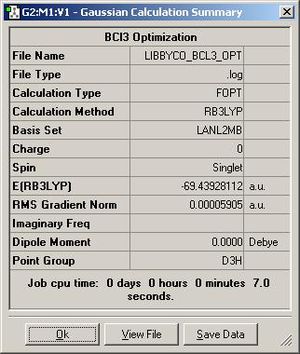
The calculation method for optimising the ground state structure of BCl3 is FOPT with the basis set being LANL2MB. This can be confirmed as a minima as both the structures which are the results of the optimisation and frequency calculations both have the same energy; this being -69.3Au
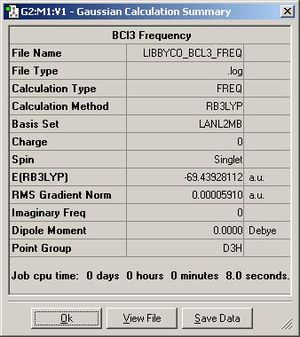
Frequency analysis ensures that the molecule that you are using is actually at the lowest possible energy; ie that the bonds are in the most stable point for the molecule to be in, in equilibrium. It is the second derivative of the energy curve, therefore ensuring that the point you have is a minimum.
The bond length if B-Cl given in literature is 175pm. The calculation shows a bond length of 186pm; this is only a difference of 9pm in total; therefore the calculations shows a good approximation for finding the bond length in molecules. The bond angle is found to be 120o in literature, which corresponds to that given as a result of the calculation.
A bond is usually formed when there are 2 opposite charges resulting in a strong force which can either be the sharing of electrons between atoms (a covalent bond ) of the donatin of electrons (the negative charge) to a positvely charged atom (an ionic bond). The electrons interact by sharing or filling different orbitals, whether they are empty of full.
The calculations took under 20 seconds to do for each one; ie the optimisation and the frequency calculation. This is a quick calculation, but this is based on the fact that I used a minimal basis set, which doesn’t probe the molecules fully. The results of both the optimisation and frequency calculations can be seen in figures 2 and 3.
The MO Diagram of BH3 with IR spectrum
The molecule of BH3 was drawn out in Gaussview (figure 4) and the energy optimisation was run as before, and ten the frequencies along with the Molecular Orbitals of the molecule were also computed. The IR frequencies are talked about below, however the molecular orbital diagram for BH3 is directly below, also showing the computed orbitals from Gassian.
The MO diagram is shown below with the computed molecular orbitals:

This shows that the computed orbitals are extremely similar to the real MO’s shown. There aren’t any majorly differences between the two, however the 3a1 molecular orbital is higher in energy in the qualitative approach than in the 2e’ orbital.. Therefore it shows that qualitative MO theory is useful, however doesn’t always predict the energy of the levels correctly, therefore it does have some limitations.
The computed IR freqencies are shown in the table below:
| Number | Form of the Vibration | Frequency (cm-1) | Intensity | Symmetry D3h Point group |
|---|---|---|---|---|
| 1 |  |
1144 | 92 | A" |
| 2 | 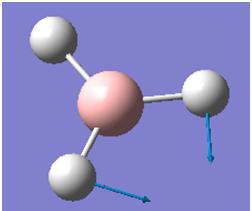 |
1203 | 12 | E' |
| 3 | 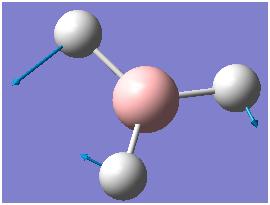 |
1203 | 12 | E' |
| 4 | 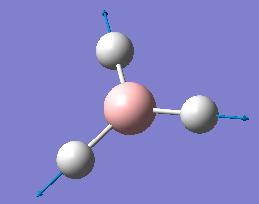 |
2598 | 0 | A' |
| 5 | 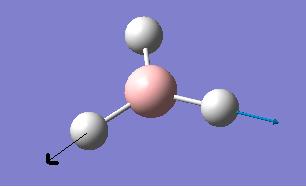 |
2737 | 103 | E' |
| 6 |  |
2737 | 103 | E' |
Isomers of Mo(CO)4L2
both the cis and trans isomer of Mo(CO)4(PCl3)2 were drawn using gaussview. These can be seen in figures 5 and 6.

Once they had been drawn out, both were sent to SCAN to be optimised using the base set of LANL2MB and the B3LYP method. They were then optimsed again using a different base set of LAN2DZ, and the resulting information can be seen in figures 7 and 8, which shows the energies of both.


As can be seen the difference in energy between the two conformers is around 0.001a.u (around 5kjmol-1) which is not a great difference. Even though the energy of the cis isomer is lower, the dipole moment of zero in the trans isomer deems it to be more stable than the cis isomer. The stability of the trans isomer will also be helped by the reduced steric effects that occur, as if there were large groups attached as ligands to the phosphorus atom, e.g phenyl groups, there would be an increased steric energy in the cis isomer, which would mean that the energy of the isomer would dramatically increase, therefore the stability of the overall complex would decrease. Therefore to change the relative ordering you could ensure that you don’t have an overly bulky ligand attached, which would make the energy of the cis isomer lower than the energy of the trans- isomer.
IR Spectra of the Two Isomers.
Once the two molecules had been optimised the frequency calculation was run to determine the IR spectra of each, so that they could be compared. For both the cis and trans isomers none of the vibrations have a negative frequency, however there are some with very low values. The values of these are shown below in the table as well as the pictures of the vibrations.
| Frequency cm-1 | Picture of vibration |
|---|---|
| 10.75 | 
|
| 17.62 | 
|
| Frequency cm-1 | Picture of vibration |
|---|---|
| 5.04 | 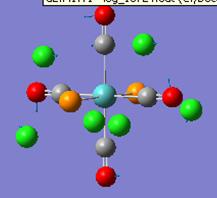
|
| 6.04 | 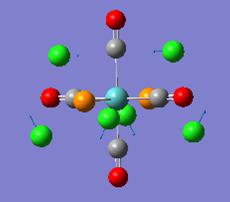
|
The cis-isomer also shows 4 vibrations at 1945, 1948, 1958 and 2023 cm-1 for the CO group. In literature1 the values given for a complex of Mo(CO)4DTH, which is a slightly different complex, however wouldn’t affect the metal-CO frequencies greatly are 1941, 1947, 1964 and 2049 cm-1. For the trans isomer there are two CO stretches of notable interest (there are 2 others, however the explanation below deals with these which are both at 1950 cm-1. This also corresponds to the literature value1 which says there is a strong signal at around 1943cm-1. At room temperature these will still occur.
As the trans molecule is symmetric there are only 2 CO stretches of significance. These are also around the same value, and so can be regarded as one stretch. The cis- isomer, however, is not symmetric and all 4 CO groups are sitting with slightly different dipoles, therefore there are 4 different CO stretches that show up on the IR.
Fuels of the Future
Ammonia borane is being investigated to see whether it can be used as a suitable ‘storage’ of hydrogen as a solid at room temperature. Below outlines the project that I have been investigating, to find out which is the most stable form of the molecule (eclipsed or staggered); and how it differs to that of ethane, both in energy and different properties such as the dipoles within the molecule, and the different bonding present.
Both forms of the compound were drawn out and the optimisation using the simplest method and base set was run. Both these returned the molecule with an energy of -82.8au, and both in the staggered conformer. This can be explained as the staggered is lower in energy due to less repulsion between the hydrogen atoms on the different groups. The resulting structure is shown below as is the output form the optimisation calculation:

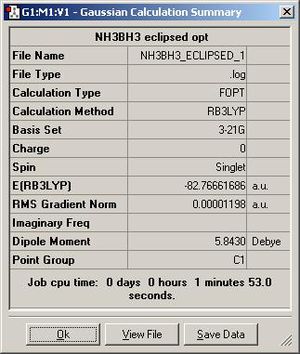
The energy of the eclipsed form of ethane is -79.4 au, and so this shows that overall the molecule of BH3NH3 is more stable at room temperature. the next thing that was compared was the dipole moment within both of the molecules. The NBO calculation was run and this resulted in the molecules shown below. Each one shows the charge at that time on a particular atom, the picture on the right shows ethan and the picture on the left shows the BH3NH3 molecule.
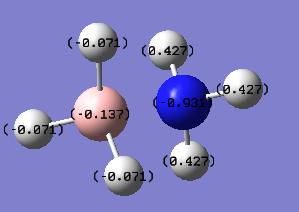
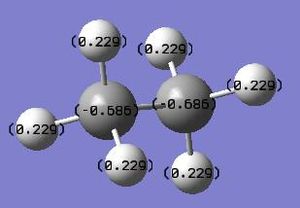
As can be seen from the charge distribution on both of the molecules, the ethane is symmetric, but the BH3NH3 has a dipole on it. It shows that the majority of the negative charge is present on the Nitrogen atom, and that out of the two atoms the boron is the more delta positive. This would prove that the ammonia borane should have a higher melting point due to the increased bonding between the nitrogen and the borane, ie the bigger dipole where the lond pair on the nitrogen atom interacts with the empty p-orbital on the borane, which will be harder to break, therfore requiring more energy, i.e. heat.
The total energy of the reactants is -703.26au, and the total energy of the products is -703.29au. This shows that the energy of the products is less than the energy of the reactants, and so the equilibrium of the reaction would be over on the side of the reactants. This shows that overall the ammonia borane molecule is stable, and so would be suitable as an alternative for hydrogen storage.
References
M.Y Darensbourg and D. J. Darensbourg, J. Chem. Ed, 1970, 47, 33
Dalton Trans., 2007, 2613 - 2626, DOI: 10.1039/b703053c
Christophorou, L. G.; Olthoff, J. K. Journal of Physical and Chemical Reference Data, Vol. 31, Issue 4, p.971 DOI: 10.1063/1.1504440
