Rep:Mod:imgettingAlder
The Diels Alder Cycloaddition
Here, the methods used previously will be applied to Diels Alder reactions. Diels Alder reactions are pericyclic and are concerted. A dieneophile interact with a diene to form σ bonds from pi bonds. The interaction of the HOMO/LUMO of the interacting species depends if the reaction is allowed or forbidden. If the HOMO of one species can interact with the LUMO of the other, the reaction is allowed. There must be significant overlap and the same symmetry. If there are substituents with π orbitals that are of the correct symmetry to interact with the π orbitals, then this can favour a particular position of attack.
Cis-butadiene and Ethylene
To start, the reactants were optimised using the AM1 semi-empirical molecular orbital method. The HOMO and LUMO of both were calculated and are shown. Ethylene is the dieneophile and cis-butadiene is the diene.
| Molecule | Molecular Orbital | Molecular Orbital Diagram | Symmetry | Energy/ Hartrees |
|---|---|---|---|---|
| Cis-butadiene | LUMO | 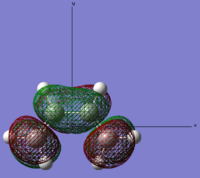 |
Symmetric | 0.01796 |
| Link to file: Media:danCISBUTADIENE-OPT.LOG | HOMO | 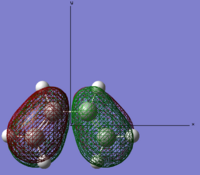 |
Anti-symmetric | -0.34455 |
| Ethylene | LUMO | 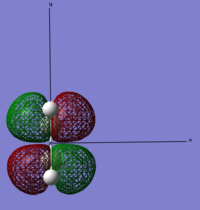 |
Anti-symmetric | 0.05284 |
| Link to file: Media:danETHYLENE.LOG | HOMO | 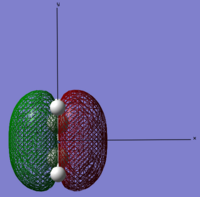 |
Symmetric | -0.38777 |
Looking at the symmetry on the MO’s we can see that the LUMO of cis-butadiene can interact with the HOMO ethylene. Therefore, this reaction is allowed. The other set of HOMO and LUMO also match but these are not π orbitals.
I calculated the transition state using the ‘Frozen coordinate’ method used earlier with the AM1 semi-empirical molecular orbital method, then this I optimised this to a higher accuracy using the B3LYP/6-31G and the following information was produced.
| Diagram | |||
| Energy/ Hartrees | -234.495 | ||
| Link to file | Media:TRANSDIELSALDER1-FROZEN-6-31GDAN.LOG | ||
| Imaginary Frequency/ cm-1 | -534 | ||
| Imaginary Frequnecy Diagram | 
|
The imaginary frequency clearly shows the concerted formation of the bonds, which is as expected. Let’s examine the HOMO and LUMO.
| Molecule | Molecular Orbital | Molecular Orbital Diagram | Symmetry | Energy/ Hartrees |
|---|---|---|---|---|
| Transition State | LUMO | [[Image:|200px]] | Symmetric | |
| HOMO | [[Image:|200px]] | Anti-symmetric |
If we calculate the energy of the optimised product we can work out the activation energy. The energy of the product is Link to file: [[Media:]]
| Activation Energy/ Hartrees | Activation Energy/ kcal/mol |
|---|---|
The value obtained is consistent with the literature value 115 KJ mol-1[1] but there is a difference and this does show that Gaussian calculation are not 100% qualitative but do give a very good approximation which was also seen earlier.
Cyclohexa-1,3-diene and Maleic Anhydride
(show reaction)
This reaction has two possible products and here we will discuss the region selectivity. The C=O bond has pi orbitals which can interact to stabilise a particular transition state. This will be shown by analysing the two possible transition states. The transition states were found using the ‘frozen coordinate’ method with the semi-empirical/AM1 method.
Again, the imaginary frequencies show the concerted bond formation nicely. We can see that the endo transition state has a lower energy than the exo transition state. If we draw the Frontier Orbitals, we can see that the endo transition state has some interaction from the C=O pi orbitals, which stablise the transition state, hence lowering its energy.
References
- ↑ Vildan Guner, Kelli S. Khuong, Andrew G. Leach, Patrick S. Lee, Michael D. Bartberger, and K. N. Houk, J. Phys. Chem. A, 2003, 107 (51), pp 11445–11459 DOI: 10.1021/jp035501w